Fast response/recovery performance of comb-like Co3O4 nanostructure
Jianan Deng,
Lili Wang,
Zheng Lou and
Tong Zhang*
State Key Laboratory on Integrated Optoelectronics, College of Electronic Science and Engineering, Jilin University, Changchun 130012, PR China. E-mail: zhangtong@jlu.edu.cn; Fax: +86 431 85168270; Tel: +86 431 85168385
First published on 28th April 2014
Abstract
A new type of comb-like architecture with a hierarchical structure was prepared via a simple and effective hydrothermal strategy in the case of Co3O4. FESEM and TEM confirm the comb-like architecture of the hierarchical structure, and that the Co3O4 nanorods with secondary nanostructures are the building units. The studies of the sensing performance demonstrate that the as-synthesized comb-like Co3O4 nanostructures show excellent catalytic activity upon exposure to CO gases in comparison with rod-like Co3O4. Importantly, the comb-like Co3O4 nanostructure-based sensor shows a fast response and recovery speed for CO gas. It is believed that the strategy of combining the hierarchical nanostructure and catalytic activity of Co3O4 nanomaterials can further provide potential for applications as real-time monitoring gas sensors.
1. Introduction
It is generally known that the properties of functional materials are not only dependent on the diverse elemental compositions but also on various surface morphologies, architecture, particle size and shape.1 In the past few years, hierarchical nanostructures, which differ from those of the corresponding bulk-state materials, have attracted significant attention owing to their unique structures, intriguing properties, and potential applications in gas sensing, photocatalysis, drug delivery systems, solar cells, electronics and so on.2 A hierarchical structure means the higher dimension of a micro- or nanostructure composed of many, low dimensional, nano-building blocks.2,3 To create such structures, man-made templates such as bubbles, colloids, polymers, surfactant, and porous anodic alumina have been widely used. Many novel structures have been achieved such as flower-like,4 branch-like,5 brush-like,6 urchin-like and so on.7 But due to the inherent structural complexity, intricate steps and stringent requirement in experimental environment and equipments, it is still hard to synthesize materials with unique multiscale structures and morphologies.P-type oxide semiconductors played very critical roles in the gas sensing field, because of the majority charge carriers and conduction paths are quite different from the n-type oxide semiconductors, the resistance often tend to be lower and with distinctive surface reactivity and oxygen adsorption are also advantageous for enhancing gas selectivity, decreasing the humidity dependence of sensor signals to negligible levels, and improving recovery speed.8 Tricobalt tetraoxide (Co3O4) is a typical and also important p-type metal oxide ceramic material, it has been long ago involved in gas sensing and many other applications, hollow rings, nanocubes, nanospheres and nanofibers have already been prepared. The gas sensing performances of Co3O4 was also has been tested and studied. Li et al.9 found Co3O4 hollow microspheres exhibited high response to ethanol at a lower operating temperature, and Wang et al.10 obtained Co3O4 nanocubes that got excellent sensitivity to xylene, Lee et al.11 reported a series of Co3O4 with different nanostructures and discussed the improvement of selectivity and sensitivity to target gases. In recent years, a numbers of nanocarbon based studies in gas sensing platforms have also been conducted, Mao and Chen et al.12 have reported a lot of gas sensor with low operation temperature and high sensitivity have been achieved such as graphene–NC hybrid and CNT–NC hybrid materials. However, the quality of nanocarbon-based sensors, e.g. reproducibility, long term stability, and false control, represents a great challenge for commercialization of sensors.13
In this work, we introduce a facile and effective method to obtain the comb-like Co3O4 assembled with hierarchical Co3O4 nanorods. Two steps are required during the process, cobalt nitrate hexahydrate was applied as the cobalt source, trisodium phosphate dodecahydrate and hydrazine hydrate were used as surface active agent. After the high temperatures and high pressures process that generated by the hydrothermal reaction, then the Co3O4 was synthesized after calcining at 400 °C for 4 h. The gas sensing properties of two materials were also recorded and studied. The comb-like hierarchical Co3O4 exhibited not only high response but also a fast response speed to CO gases.
2. Experimental
2.1. Synthesis of material
All the reagents were of analytical grade and used without further purification. Trisodium phosphate dodecahydrate and cobalt nitrate hexahydrate were purchased from Sinopharm Chemical Reagent Co. (Shanghai, China) and Guangfu Technology Development Co., LTD (Tianjin, China), respectively.2.2. Characterizations
The morphologies of the samples and energy dispersive X-ray (EDX) analysis were obtained on a XL 30 ESEM FEG field emission scanning electron microscope (FESEM). X-ray diffraction patterns (XRD) were recorded by a Rigaku D/Max-2550 diffractometer with Cu Kα radiation (40 kV, 200 mA) in the range of 20–80°(2θ) at a scanning rate 6° min−1. The transmission electron microscopic (TEM) and high resolution transmission electron microscopic (HRTEM) images were performed on a JEOL JEM-3010 TEM microscope.2.3. Fabrication and measurement of gas sensor
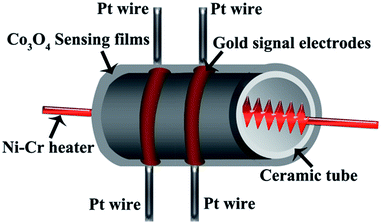
0.1 g of the comb-like hierarchical Co3O4 were dispersed in 0.25 g of deionized water and transferred to an agate mortar. After grinding for 1 h, the resulting mixture formed a black paste. The main body of a gas senor was a ceramic tube with two Au signal electrodes, each electrode equipped with two Pt wires and coated with above mentioned paste. Fig. 1 is a diagrammatic sketch of the as-fabricated sensor that reveals the structure of the sensor. The heating source of the indirect-heated gas sensor comes from a Ni–Cr heating wire that placed in the center of the ceramic tube, the gas sensor is used as a resistor in the circuit. The rod-like Co3O4 sensors are prepared for comparison with same method. There are some resemblances of the parameters and manufacture details between the sensor we made and that reported in the literature.15–17The RQ-2 Intelligent testing platform (China) was introduced to keep the record of the gas sensors' sensing performances. The sensitivity for p-type semiconductors was defined as S = Rg/Ra, S is short for sensitivity, where Ra and Rg are the numerical value of electrical impedance in air and in reducing gases, respectively. However, for p-type semiconductors, the definition of the sensors' sensitivity was just the opposite. The response and recovery time were defined as the time taken by the sensor to achieve 90% of the total resistance variation in the case of adsorption and desorption, respectively.14
3. Results and discussions
3.1. Structural and morphological characteristics
Fig. 2 shows the XRD patterns of as synthesized comb-like Co3O4 hierarchical nanostructures and the rod-like Co3O4 nanostructures. The intensities of the sharp diffraction peaks indicated that the product has good crystallinity. All of the diffraction peaks (Fig. 2(a) and (b)) in the XRD patterns can be indexed as a hexagonal phase of Co3O4 (JCPDS card 43-1003). No other diffraction peaks related to impurities are observed. | ||
| Fig. 2 X-ray diffraction (XRD) pattern of the products: (a) comb-like Co3O4 hierarchical nanostructure, (b) rod-like Co3O4 nanostructure. | ||
Fig. 3(a) and (b) shows the FESEM images of the as-synthesized samples, it can be obviously observed that the comb-like hierarchical Co3O4 is successfully achieved, the comb-like hierarchical structure is consist of a number of hierarchical Co3O4 nanorods with diameters ranging from 100 to 200 nm and lengths ranging from 3 to 4 μm. The nanorods sticked together in the center parallelly (Fig. 3(c)) and formed a comb-like hierarchical structure. From the close observation to the end of the nanorods, (Fig. 3(d)) a clear view can be seen that the surface of the nanorods are pretty rough and thousands of thorns densely and randomly came out of the main Co3O4 nanorods. The rod-like Co3O4 nanostructures were also prepared for comparison, Fig. 3(e) and (f) is the FESEM images of the rod-like Co3O4, the surface is rather smooth in comparison with the comb-like hierarchical Co3O4, no dense secondary structures are found on the surface of the nanorods. The diameter of rod-like Co3O4 is a little bit larger than that of the comb-like hierarchical Co3O4, which is ranging from 200 to 300 nm but the lengths of this two materials are quite the same which are about several micrometer. Fig. 3(g), (h), (j) and (k) are the TEM images of the two materials, through the TEM images more details could be observed which in coincidence with the results we obtained in the SEM images, and confirmed that the comb-like Co3O4 hierarchical nanostructure was successfully achieved. Moreover, the lattice fringes are clearly visible with a spacing of 0.47 nm (Fig. 3(i)) and 0.24 nm (Fig. 3(l)), which are in a good agreement with the spacing of the (111) and (311) planes of Co3O4, respectively.
3.2. CO sensing properties
Because of the substantial differences in morphologies and crystal structures, only by cooperating with proper working temperature can the sensing materials exhibit their best performance in the sensing application. So choosing an optimal operating temperature is not only necessary but also critical to a successful gas sensor.18 The responses of the comb-like Co3O4 and rod-like Co3O4-based sensors to 50 ppm CO gas at different working temperatures are given in Fig. 4. The working temperatures are ranging from 120 to 240 °C, and each plot formed its own peak at 180 °C, on the left side of the peak, the response values increases correspondingly as the working temperature rises, and after reached the peak value which is 8.6 to the comb-like Co3O4 and 4.2 to the rod-like Co3O4, the plots start to go down, which means that two materials both reach their maximum response at 180 °C to 50 ppm of CO. Therefore, we choose 180 °C as our working temperature to proceed with the subsequent detections. | ||
| Fig. 4 The responses to 50 ppm CO gas of the comb-like Co3O4 hierarchical nanostructure (black dot) and rod-like Co3O4 (red dot)-based sensor at different working temperatures. | ||
Gas sensors that have been put into application fields always facing different surrounding environments and situations, in the industrial production area multiple gases could sometimes be involved. Therefore, the good selectivity to target gas is always one of the key factors to the gas sensors. The comparisons of the selectivity between comb-like Co3O4 and rod-like Co3O4-based sensors to 50 ppm various gases (CO, H2, H2S, C2H2, C2H4) at 180 °C was measured and showed in Fig. 5. The response of comb-like Co3O4 and rod-like Co3O4 based sensors to CO, H2, H2S, C2H2, C2H4 were 8.6, 3.1, 2.9, 2.6, 1.7 and 4.2, 1.7, 1.4, 1.3, 1.2 respectively. The comb-like Co3O4-based sensor apparently got not only higher response but also better selectivity to CO gas than that of the rod-like based one.
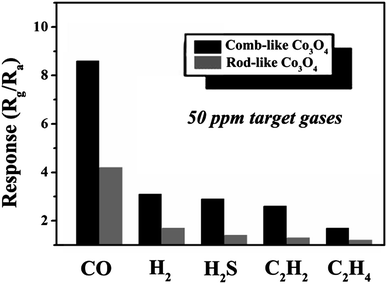 | ||
| Fig. 5 Response of the comb-like Co3O4 (black) and the rod-like Co3O4 (grey) versus 50 ppm of various gases at 180 °C, respectively. | ||
Fig. 6(a) and (b) shows the dynamic response and recovery curves of the comb-like Co3O4 and the rod-like Co3O4 based sensors to 1–100 ppm CO at 180 °C. The two materials are both showing response to CO gas, the response value varies as the concentration changes and thus the curves exhibit a stepwise distribution. Although both comb-like hierarchical Co3O4 and the rod-like Co3O4 show responds to CO gas, but the former one exhibits much more response to CO than that of the latter one, no matter at low or high concentration levels. The responses of rod-like Co3O4-based sensor to 1–100 ppm CO were 1.5, 2.0, 2.6, 3.3, 4.2 and 5.1, respectively (Fig. 6(b)). The response of comb-like Co3O4-based sensor to 1–100 ppm CO were 2.3, 3.8, 5.3, 6.7, 8.6 and 11.6, respectively (Fig. 6(a)), which were significantly improved and about 2 times higher than that of the prior material.
 | ||
| Fig. 6 Dynamic response and recovery curves of (a) the comb-like Co3O4 and (b) the rod-like Co3O4 based sensors to 1–100 ppm CO at 180 °C. | ||
Fig. 7(a) displays the plots of sensing response versus the CO concentration when the sensors based on two Co3O4 samples were exposed to CO with the concentration ranging from 1 to 1000 ppm. As the CO concentration increased, the responses of the two sensors also increased. Besides, the CO responses for two sensors were linear between 1 and 20 ppm (Fig. 7(b), inset). This indicates that the comb-like Co3O4 nanostructures are more beneficial for the exploration of low concentrations of CO gas.
 | ||
| Fig. 7 The relationship curves of gas responses and concentration at 180 °C: (a) 1–1000 ppm, (b) 1–20 ppm. | ||
Fig. 8(a) shows the enlarged response and recovery curves of two sensors, there are obvious differences between the two sensors in response and recovery speed. The remarkable point is the ultra-fast response and recovery of comb-like Co3O4-based sensor. The response times (τres) upon exposure to 10, 20, and 50 ppm of CO are 0.9 s, 0.7 s, and 0.5 s, respectively. The corresponding recovery times (τrecov) are all approximately 2.3 s, respectively. In contrast, under the same conditions, the τres of rod-like Co3O4 based sensor are approximately 5 s, and the τrecov are 19.7, 17.5 and 15.0 s, respectively. Fig. 8(b) shows the response and recovery speed in comparison of comb-like Co3O4 and rod-like Co3O4 towards 1–100 ppm CO. The τres is about 2 s for comb-like Co3O4 based sensor to 1 ppm CO, whereas rod-like Co3O4-based sensor is 11 s (Fig. 8(b)). Accordingly, the τrecov of comb-like Co3O4 based sensor is about 4 s, but it takes 25 s for rod-like Co3O4. Such unusual response and recovery behavior has not been observed in conventional nanomaterials (Table 1).19–22 It clearly demonstrated the great advantage in real-time monitoring of the comb-like hierarchical Co3O4.
3.3. Gas sensing mechanism of comb-like Co3O4 hierarchical nanostructures
Such good performances of comb-like Co3O4 hierarchical nanostructures can be qualitatively explained by a well-known theory as follow. For semiconductor oxide gas sensor, the response is measured by calculating the variation of resistance that before and after the sensor was exposed to target gases. This is usually attribute to the surface oxygen that adsorbed on the surface of the semiconductor, unlike n-type sensor, the major carriers of p-type oxide semiconductor are holes,8,23,24 so in this work, the CO sensing process is based on the changes in the resistance of the Co3O4 which is controlled by the CO species and the amount of the chemisorbed oxygen on the surface.Fig. 9 showed the schematic diagrams on the gas sensing mechanism of comb-like Co3O4, the free electrons of the Co3O4 were captured by the adsorbed oxygen molecules and then the oxygen further transformed to be oxygen anion such O2− or O−.25,26 For comb-like Co3O4 with a hierarchical nanostructure, it is well known that hierarchical nanostructures with large surface area and well-aligned porous structures have been reported.3,27 The surface area values of the comb-like Co3O4 and rod-like Co3O4 are 30.0 m2 g−1 and 7.7 m2 g−1, respectively. When comb-like Co3O4 are putted in a test gas, the gas would be easy to diffused to throughout particles, resulting in increase of the effective surface area. Thus, high response was obtained. However, the comb-like Co3O4 based sensor not only exhibited greater response but also faster response/recovery speed than the rod-like Co3O4 one, this result can be ascribed to the advantage of the different morphology, i.e., perfect comb-like and hierarchical nanostructures.28,29 Referring to the FESEM and TEM images in Fig. 3(c) and (h), it can be clearly observed that the comb-like structure well organized and orderly assembled the hierarchical Co3O4 nanorods. This organized and orderly assembled hierarchical structure makes the initial Co3O4 nanorods less agglomerated and also provides more interspace on the surface and interior which enhances the gas diffusion speed (Fig. 9), so the target gases could come and go much more freely which significantly increases the response and recovery speed. Compare to the rod-like Co3O4 nanostructure as shown in Fig. 3(f), it has a solid interior structures and the gas diffusion toward the entire sensing surface is hampered. Relatively long response speed was obtained. Therefore, the comb-like Co3O4 hierarchical nanostructures achieved great enhancement both in gas response and response/recovery speed.
 | ||
| Fig. 9 Schematic diagrams on the gas sensing mechanism of comb-like Co3O4 hierarchical nanostructures. | ||
4. Conclusions
The comb-like Co3O4 hierarchical nanostructure was successfully prepared through a simple combination of hydrothermal process and calcination by controlling the reaction time and the amount of surfactant. The gas sensing properties indicated that it showed not only better response but also outstanding response and recovery speed to CO at 180 °C compare with the Co3O4 nanorods. Furthermore, it also possesses a good selectivity among various interfering gases, all the above mentioned features make it a very promising candidate for future commercial and industrial real-time CO detecting or monitoring system and potential application in protecting human lives and property.Acknowledgements
This research work was financially supported by the Natural Science Foundation Committee (NSFC, Grant no. 51102109) and the Program for Chang Jiang Scholars and Innovative Research Team in University (no. IRT1017).Notes and references
- E. V. Shevchenko, M. I. Bodnarchuk, M. V. Kovalenko, D. V. Ta-lapin, R. K. Smith, S. Aloni, W. Heiss and A. P. Alivisatos, Adv. Mater., 2008, 20, 4323 CrossRef CAS.
- Y. Zhao and L. Jiang, Adv. Mater., 2009, 21, 3621 CrossRef CAS.
- J. H. Lee, Sens. Actuators, B, 2009, 140, 319 CrossRef CAS PubMed.
- L. L. Wang, T. Fei, Z. Lou and T. Zhang, ACS Appl. Mater. Interfaces, 2011, 3, 4689 CAS.
- J. N. Deng, B. Yu, Z. Lou, L. L. Wang, R. Wang and T. Zhang, Sens. Actuators, B, 2013, 184, 21 CrossRef CAS PubMed.
- Z. Lou, F. Li, J. N. Deng, L. L. Wang and T. Zhang, ACS Appl. Mater. Interfaces, 2013, 5, 12310 CAS.
- Z. Lou, Y. L. Feng, X. W. Liu, L. L. Wang and T. Zhang, Biomed. Eng., 2012, 24, 99 CAS.
- H. J. Kim and J. H. Lee, Sens. Actuators, B, 2014, 192, 607 CrossRef CAS PubMed.
- Q. Jiao, M. Fu, C. You, Y. Zhao and H. Li, Inorg. Chem., 2012, 51, 11513 CrossRef CAS PubMed.
- C. Sun, X. Su, F. Xiao, C. Niu and J. Wang, Sens. Actuators, B, 2011, 157, 681 CrossRef CAS PubMed.
- K. I. Choi, H. R. Kim, K. M. Kim, D. W. Liu, G. Z. Cao and J. H. Lee, Sens. Actuators, B, 2010, 146, 183 CrossRef CAS PubMed.
- S. Mao, G. H. Lu and J. H. Chen, J. Mater. Chem. A, 2014, 2, 5573 CAS.
- S. M. Cui, S. Mao, G. H. Lu and J. H. Chen, J. Phys. Chem. Lett., 2013, 4, 2441 CrossRef CAS.
- Y. Z. Shao, J. Sun and L. Gao, J. Phys. Chem. C, 2009, 113, 6566 CAS.
- L. L. Wang, Z. Lou, T. Fei and T. Zhang, J. Mater. Chem., 2011, 21, 19331 RSC.
- L. L. Wang, T. Fei, J. N. Deng, Z. Lou, R. Wang and T. Zhang, J. Mater. Chem., 2012, 22, 18111 RSC.
- L. L. Wang, H. M. Dou, Z. Lou and T. Zhang, Nanoscale, 2013, 5, 2686 RSC.
- C. Zhang, A. Boudiba, M. G. Olivier, R. Snyders and M. Debliquy, Sens. Actuators, B, 2012, 175, 53 CrossRef CAS PubMed.
- M. Hjiri, L. El Mira, S. G. Leonardi, A. Pistone, L. Mavilia and G. Neri, Sens. Actuators, B, 2014, 196, 413 CrossRef CAS PubMed.
- C. R. Michel and A. H. M. Preciado, Sens. Actuators, B, 2014, 197, 177 CrossRef CAS PubMed.
- D. Patil, P. Patil, V. Subramanian, P. A. Joy and H. S. Potdar, Talanta, 2010, 81, 37 CrossRef CAS PubMed.
- T. Krishnakumar, R. Jayaprakash, N. Pinna, N. Donato, A. Bonavita, G. Micali and G. Neri, Sens. Actuators, B, 2009, 143, 198 CrossRef PubMed.
- Y. M. Sui, Y. Zeng, L. L. Fu, W. T. Zheng, D. M. Li, B. B. Liu and B. Zou, RSC Adv., 2013, 3, 18651 RSC.
- L. L. Wang, Z. Lou, T. Fei and T. Zhang, Sens. Actuators, B, 2012, 161, 178 CrossRef CAS PubMed.
- P. Rai, R. Khan, S. Raj, S. M. Majhi, K. K. Park, Y. T. Yu, I. H. Lee and P. K. Sekhar, Nanoscale, 2014, 6, 581 RSC.
- S. C. Chang, J. Vac. Sci. Technol., 1980, 17, 366 CrossRef CAS.
- H. R. Kim, K. I. Choi, J. H. Lee and S. A. Akbar, Sens. Actuators, B, 2009, 136, 138 CrossRef CAS PubMed.
- X. Chen, Z. Guo, W. H. Xu, H. B. Yao, M. Q. Li, J. H. Liu, X. J. Huang and S. H. Yu, Adv. Funct. Mater., 2011, 21, 2049 CrossRef CAS.
- L. L. Wang, Z. Lou, T. Zhang, H. T. Fan and X. J. Xu, Sens. Actuators, B, 2011, 155, 285 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |