Tetrahydropyranyl ether (THPE) formation in hydroxyl group protection and conversion to other useful functionalities
Abstract
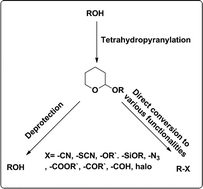
This short review highlights the various methods of formation of tetrahydropyranyl ethers (THPEs) as a method for the protection of simple alcohols as well as a diverse range of complex molecules using a variety of reagents and reaction conditions, i.e., acid catalyst, heterogeneous catalyst and neutral reagent mediated reactions. We also discuss their direct conversion to other useful functionalities.

 Please wait while we load your content...
Please wait while we load your content...