One-step vapor diffusion synthesis of uniform CdS quantum dots/reduced graphene oxide composites as efficient visible-light photocatalysts
Min Fua,
Qingze Jiaoab and
Yun Zhao*a
aSchool of Chemical Engineering and the Environment, Beijing Institute of Technology, Beijing 100081, China. E-mail: zhaoyun@bit.edu.cn; Fax: +86 10 68918979; Tel: +86 10 68918979
bSchool of Chemical Engineering and Materials Science, Beijing Institute of Technology, Zhuhai 519085, China
First published on 12th May 2014
Abstract
CdS quantum dots (QDs)/reduced graphene oxide (RGO) composites were synthesized through a one-step vapor diffusion process in the presence of ethylene glycol. The in situ growth of CdS QDs and the reduction of graphene oxide (GO) were completed simultaneously. Fourier transform infrared spectra, X-ray diffraction patterns, X-ray photoelectron spectroscopy and Raman spectroscopy confirmed the reduction of GO. Electron microscopy indicated uniform CdS QDs with size around 4–7 nm were well distributed on the RGO sheets. The transient photocurrent response, electrochemical impedance spectroscopy and diffuse reflectance UV-visible spectra of CdS QDs/RGO composites and CdS were tested to explain the role of RGO for the photocatalytic reaction. As-obtained composites exhibited better photocatalytic properties than pure CdS under visible light irradiation. The influence of different contents of GO on photocatalytic performance was also investigated. A possible photocatalytic mechanism of CdS QDs/RGO composites was proposed.
1. Introduction
With the rapid development of science and technology, water pollution is becoming increasingly severe. In recent decades, semiconductor materials have been of great research interest due to their novel properties and potential photocatalysis applications.1,2 As a well-known II–VI semiconductor, CdS has been extensively studied due to its relatively narrow bandgap (2.40 eV) and size-dependent electronic and optical properties.3,4 Compared with their wide bandgap counterparts, CdS are promising photocatalytic materials for the conversion of solar energy into chemical energy under visible-light irradiation. CdS have shown many commercial applications including solar cells, photoelectronic devices, biological sensors and photocatalysis.5–14However, there are a few issues that restrict their photocatalysis application. For instance, CdS particles are easy to aggregate, resulting in a relatively smaller surface area.15 Also, the rapid recombination of photogenerated electron–hole pairs and the photo-corrosion phenomenon deteriorate their photocatalytic performance.16 Many attempts have been explored to improve their photocatalytic activity and photochemical stability, such as synthesis of CdS quantum dots (QDs)17 and combination with other components, including semiconductors or carbon based materials.18–23
QDs have attracted tremendous interest in recent years, due to their unique physical and chemical properties. The two existent general strategies of QDs preparation are organometallic synthesis and aqueous synthesis.24 However, these approaches usually suffer from high temperature, which limits their practical applications. Therefore, exploring of simple methods for the size controlled synthesis of CdS QDs is still of scientific importance. The vapor diffusion approach is one kind of method which can be used for controllable preparation of unique micro–nano structure materials.25 In vapor diffusion reaction, the precipitating agent and metal salt solution are placed separately. The slow decomposition of precipitating agent guarantees the controllable growth of precipitation.
Combination with other components has been demonstrated to be another strategy to overcome above drawbacks. Among the promising carbon materials, graphene possesses single-atom thickness, abundant sp2-hybridized two-dimensional carbon network with extraordinary conductivity and exceptional physical properties.26–29 Graphene with large specific surface area, acting as outstanding electron acceptors and highly conductive optoelectronic scaffolds, has found its applications in photocatalysis and photovoltaic conversion.30
There have been several reports in the synthesis and application of graphene–CdS composites until now.31–33 Cao et al. prepared graphene–CdS through a solvothermal method at the temperature of 180 °C in dimethyl sulfoxide.31 Li et al. synthesized CdS clusters–graphene composites using graphene oxide (GO) as the support and cadmium acetate as the CdS precursor, and investigated their hydrogen evolution performance.32 Chang et al. prepared CdS QDs sensitized graphene by a two-step reaction. Firstly, graphene was functionalized with pyrenebutyrate. Secondly, CdS QDs deposited and grew on pyrenebutyrate functionalized graphene.33 However, in these works, high temperature or complicated operation were usually needed. Hence, it is urgent for us to synthesize CdS QDs/graphene composites by a simple, low temperature method.
In this paper, CdS QDs/reduced graphene oxide (RGO) composites were synthesized by a one-step vapor diffusion method. Methylene blue (MB) was used as a model organic contaminant, and the photodegradation of MB by as-obtained composites was investigated.
2. Experimental
Natural flake graphite powder (325 mesh) was purchased from Beijing Creative Biological Engineering Materials Co. Ltd. All other reagents and solvents were purchased from Beijing Chemicals. All chemicals were of analytical grade and used without further purification.2.1 Preparation of GO
GO was synthesized from natural flake graphite powder by a modified Hummers method.34 The detailed processing is described as below: 1 g of graphite and 0.5 g of NaNO3 were mixed with 23 ml of H2SO4 (98%) in a 250 ml three-necked flask at 0 °C. The mixture was then stirred for 0.5 h. 3 g of KMnO4 was added to the suspension carefully with vigorous stirring. After that, the mixture was stirred at 15 °C for 2 h. Then, the reaction temperature was increased to 40 °C and kept for additional 1 h. 46 ml of H2O was slowly added to the pasty mixture with vigorous agitation, and the reaction temperature was rapidly increased to 98 °C. Finally, 10 ml of 10% H2O2 solution was dropped to the mixture and the temperature was kept at 98 °C for 1 h. The mixture was centrifuged and rinsed with 10% of HCl solution and deionized water thoroughly. The as-obtained graphite oxide was re-dispersed in deionized water with ultrasonication for 2 h, then GO was obtained. In order to remove unexfoliated particles, the GO slurry was then subjected to centrifugation at 4000 rpm for 10 min. For further purification, GO was dialyzed for one week to remove residual salts and acids. The schematic diagram of preparation for GO is shown in Fig. 1.2.2 Preparation of CdS QDs/RGO composites
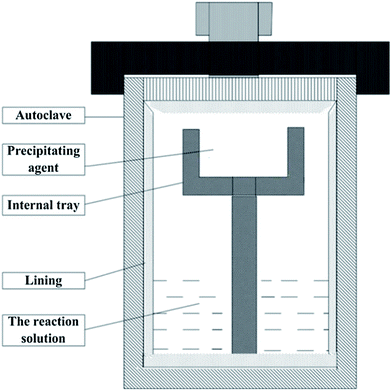
The detailed process is described as follows: 60 mg of GO was dispersed in 40 ml of ethylene glycol (EG) with ultrasonication for 2 h. EG was acted simultaneously as the solvent and the reducing agent. Subsequently, 0.266 g of Cd(CH3COO)2·2H2O was added into the above solution with magnetic stirring, the resulting solution was stirred for an additional 30 min. After substantial stirring, the mixture was transferred into a 60 ml Teflon-lined autoclave and 0.15 g of C2H5NS was placed in the internal tray. The autoclave was sealed and maintained at 80 °C for 12 h, then naturally cooled to room temperature. The precipitate was collected, washed, and dried under vacuum at 60 °C for 8 h. In order to investigate the influence of different amounts of GO on photocatalytic performance, 30, 90 mg of GO were used respectively, to prepare CdS QDs/RGO composites. The pure CdS were also prepared. According to the content of GO in the composites, the products were labeled as pure CdS, composites (30), composites (60) and composites (90), respectively. The schematic diagram of the reaction apparatus is shown in Fig. 2.2.3 Characterization
The field emission scanning electron microscopy (FESEM) was performed with a Hitachi S-4800 microscope operated at 15 kV. Transmission electron microscopy (TEM) was carried out using a Hitachi HT7700 microscope and high resolution transmission electron microscopy (HRTEM) was performed with a JEM-2010 microscope operated at 150 kV. X-ray diffraction (XRD) patterns were recorded on a X-ray diffractometer (Ultima IV) at 40 kV and 150 mA with Cu Kα radiation. The diffraction data was recorded for 2θ angles between 5° and 80°. Fourier transform infrared (FTIR) spectra were recorded on a Bruker VECTOR 22 spectrometer in the frequency range of 4000–500 cm−1. The chemical states of samples were investigated by X-ray photoelectron spectroscopy (XPS, PHI 5300X). Raman spectra were taken using a Micro-Raman spectrometer system (Renishaw RM 2000). Diffuse reflectance UV-visible spectra of samples in the wavelength range of 200–800 nm were measured using a UV-vis scanning spectrophotometer (Hitachi U-3010), while BaSO4 was used as a reference. All photocurrent and electrochemical experiments were performed with a CHI 660 electrochemical workstation and conducted in a nitrogen-purged 0.5 M Na2SO4 electrolyte solution at pH of 7, using the three-electrode setup (particles-coated ITO electrode as the photoanode, Hg/Hg2Cl2 as the reference electrode, and a platinum wire as the counter electrode). The photocurrent was measured with a bias voltage of 0.25 V. The photocurrent was measured for each switch-on/off event by using a xenon lamp (450 W) with a 460 nm cut-off filter. Electrochemical impedance spectroscopy (EIS) was measured in 0.1 M KCl solution containing 5 mM K3[Fe(CN)6] by applying an ac amplitude of 5 mV under an open circuit potential in a frequency range from 180 kHz to 0.05 Hz. UV-vis spectrophotometer (756 PC) was purchased from Shanghai Spectrum Instruments Co., Ltd.2.4 Photocatalytic degradation of MB under visible light
Photocatalytic activity of the samples was determined by the degradation of MB under visible light irradiation at room temperature. A 200 W Xe-illuminator was used as a light source and set about 10 cm from the reactor. 50 mg of different samples were added into 500 ml of 5 mg l−1 MB aqueous solution. Before irradiation, the solutions were stirred in the dark for 30 min in order to reach the adsorption–desorption equilibrium. During the given time intervals, the photoreacted solution (3 ml) was extracted and then was separated by membrane filter (pore size 0.22 μm) to remove essentially all the photocatalyst particles. Finally, a UV-vis spectrophotometer was used to determine the degradation efficiency (C/C0).3. Results and discussion
3.1 Morphology of GO and CdS QDs/RGO composites
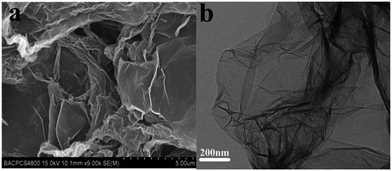
The FESEM and TEM images of GO are shown in Fig. 3. For the FESEM image (Fig. 3a), it was clearly seen that the pure GO had a flaky structure with a very smooth surface.35 The TEM image of GO shown in Fig. 3b indicated that GO were flat and transparent, with some wrinkles and folding on the surface and edge.In our experiment, 0, 30, 60, 90 mg of GO were used to prepare pure CdS and different CdS QDs/RGO composites, respectively. According to the amount of GO in the synthesis of composites, the products were labeled as pure CdS, composites (30), composites (60) and composites (90), respectively. HRTEM was employed to evaluate the optimum ratio between CdS QDs and RGO. It can be seen from Fig. 4a that the morphology of pure CdS was not uniform and the size of CdS was so large that it can not be regarded as QDs. However, the morphologies of all three CdS QDs/RGO composites were uniform and the distribution of CdS QDs on RGO surface became looser with increasing the amount of GO. The optimum ratio between CdS QDs and RGO can be determined based on both HRTEM images and photocatalytic properties.
 | ||
| Fig. 4 HRTEM images of (a) pure CdS, (b) CdS QDs/RGO composites (30), (c) CdS QDs/RGO composites (60), (d) CdS QDs/RGO composites (90). | ||
Fig. 5 shows TEM and HRTEM images of CdS QDs/RGO composites (60). As shown in Fig. 5a and b, many CdS QDs with size around 4–7 nm were dispersed on a whole RGO sheet uniformly. There was no large area of the RGO sheets without CdS decoration, no apparent aggregation of CdS QDs, and no individual CdS QDs outside of the RGO sheets. CdS QDs were directly decorated on the RGO sheets, and no molecular linkers were needed to bridge the QDs and the RGO matrices. The loading of CdS QDs prevented RGO sheets from being restacked during the reduction. RGO sheets also inhibited the agglomeration of CdS QDs, which were beneficial to the formation of QDs.36–40 The good distribution of CdS QDs on RGO sheets guaranteed their efficient photocatalytic properties.41 The lattice fringe of RGO and CdS were both observed in Fig. 5c. The lattice spacing of RGO was 0.35 nm, lower than that of GO, suggesting the removal of the oxygen-containing functional groups.42 The lattice spacing of CdS QDs was 0.336 nm, which agreed well with the basal spacing of (111) lattice planes. The selected area electron diffraction (SAED) pattern (inset in Fig. 5d) of CdS QDs showed well-defined diffraction spots, confirming the polycrystalline nature of CdS QDs.43 All of the above analysis confirmed the successful in situ growth of CdS QDs on the RGO sheets.
 | ||
| Fig. 5 TEM (a and b), HRTEM (c and d) images and SAED pattern (inset in d) of CdS QDs/RGO composites (60). | ||
The interaction between RGO and CdS was probably chemical bond. Firstly, the surface of GO was decorated with oxygen-containing functional groups, therefore the transition metal cadmium ions were easy to coordinate with oxygen on the surface of GO and serve as nucleation precursors.44–46 Secondly, the slow decomposition of C2H5NS generated H2S, which diffused into the reaction solution, generated S2− and precipitated Cd2+ on the surface of GO gradually, resulting in the formation of CdS quantum dots. At the same time, GO was reduced by ethylene glycol. As a result, GO was simultaneously reduced to RGO, accompanied with the in situ growth of CdS on the RGO support during the reaction. Also, some CdS grew on the nonfunctionlized sites of the surface of GO, the interaction between the two components was probably van der Waals force. However, the precise components interaction mechanism is not very clear now. This needs to be studied systematically from both theoretical and experimental aspects, which will be a key to guide the future work of researchers and promote the development of graphene–inorganic composites.47
3.2 Structure of CdS QDs/RGO composites
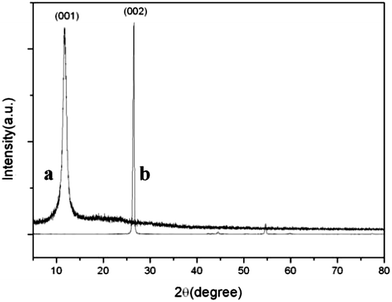
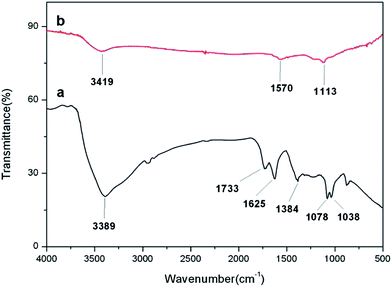
XRD was employed to investigate the phase and structure of the synthesized samples. Fig. 6 shows the XRD patterns of graphite and GO. The sharp (002) peak of pristine graphite at 2θ = 26.7° indicated a highly organized crystal structure, while the GO pattern showed a (001) characteristic peak at 2θ = 11.8°. As shown in Fig. 7, three relatively strong diffraction peaks appeared at 2θ = 26.5°, 43.7°, 51.8°, correspond to the (111), (220) and (311) planes of the cubic structure of the CdS QDs, respectively (JCPDS no. 65-2887). It was interesting to note that there was no characteristic peak of RGO in the CdS QDs/RGO composites (Fig. 6). This may be attributed to the fact that the (111) diffraction peak of CdS at 2θ = 26.5° was so strong that it may cover up the relatively weak characteristic peak of RGO.48 And the disappearance of (001) diffraction peak of GO confirmed its reduction and the recovery of ordered graphitic crystal structure.FTIR spectra of GO and CdS QDs/RGO composites are shown in Fig. 8. As shown in Fig. 8a, the peaks of GO appeared at 3389, 1733, 1625, 1384, 1078 and 1038 cm−1 were due to the vibration and deformation bands of O–H, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations from carbonyl groups, C
O stretching vibrations from carbonyl groups, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C configurable vibrations from the aromatic zooms, C–OH stretching vibrations, C–O vibrations from epoxy groups, C–O vibrations from alkoxy groups, respectively.49 However, most of these peaks related with the oxygen-containing functional groups vanished in the FTIR spectrum of the CdS QDs/RGO composites (Fig. 8b), revealing that these oxygen containing functional groups were almost removed in the reaction. New peaks that appeared at 1570 and 1113 cm−1 were attributed to the skeletal vibration of the RGO sheets.50–52
C configurable vibrations from the aromatic zooms, C–OH stretching vibrations, C–O vibrations from epoxy groups, C–O vibrations from alkoxy groups, respectively.49 However, most of these peaks related with the oxygen-containing functional groups vanished in the FTIR spectrum of the CdS QDs/RGO composites (Fig. 8b), revealing that these oxygen containing functional groups were almost removed in the reaction. New peaks that appeared at 1570 and 1113 cm−1 were attributed to the skeletal vibration of the RGO sheets.50–52
XPS was also employed to analyze GO and the CdS QDs/RGO composites. Fig. 9a shows the C1s peaks of GO, which consists of two main components arising from C–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (the non-oxygenated ring C, 284.6 eV) and C–O (hydroxyl and epoxy, 286.5 eV) groups and two minor components from C
C (the non-oxygenated ring C, 284.6 eV) and C–O (hydroxyl and epoxy, 286.5 eV) groups and two minor components from C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (the carbonyl C, 288.3 eV) and O–C
O (the carbonyl C, 288.3 eV) and O–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (the carboxylate C, 290.3 eV) groups.53,54
O (the carboxylate C, 290.3 eV) groups.53,54
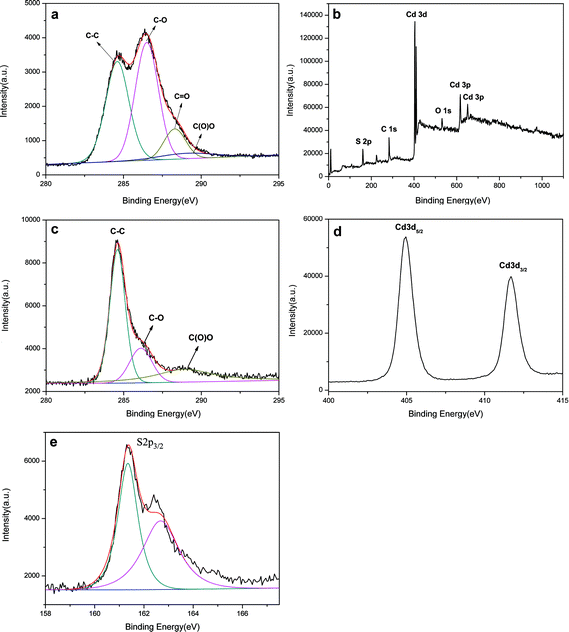 | ||
| Fig. 9 XPS spectra of the (a) C1s of GO, (b) survey scan, (c) C1s region, (d) Cd3d region, (e) S2p region of the CdS QDs/RGO composites. | ||
The wide scan XPS spectrum (Fig. 9b) of the CdS QDs/RGO composites shows photoelectron lines at a binding energy of 284.6, 531.5, 159.1 and 403.2 eV attributed to C1s, O1s, S2p and Cd3d, respectively. Compared with GO, the oxygen content of the CdS QDs/RGO composites decreased rapidly, suggesting a remarkable reduction after the reaction (Fig. 9c). Fig. 9d shows the spectrum of Cd3d region. The peaks of Cd3d5/2 and Cd3d3/2 were located at 405.0 and 411.7 eV, respectively. In Fig. 9e, the peaks at 161.3 and 162.7 eV were ascribed to the S2p3/2 level.
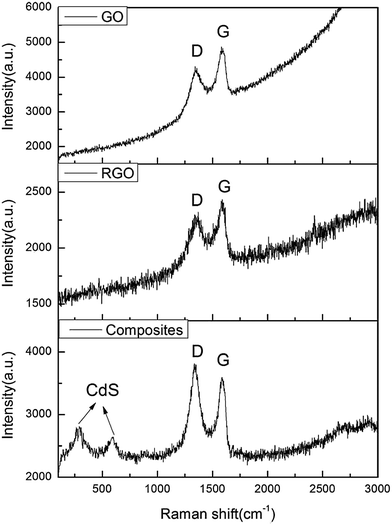
Raman spectroscopy is one of the most sensitive and informative techniques to characterize disorder in sp2 carbon materials.55 Fig. 10 shows the typical Raman spectra of GO, RGO and CdS QDs/RGO composites. The D peak arises from the disruption of the symmetrical hexagonal graphitic lattice as a result of internal structural defects, edge defects, and dangling bonds. The G peak is the response of the in-plane stretching motion of symmetric sp2 C–C bond.56 In the Raman spectrum of GO, the D and G peaks were observed at 1346 and 1584 cm−1, respectively. After GO was reduced to RGO, similar peaks appeared at 1352 and 1586 cm−1, respectively. However, the relative intensity of D/G was 0.886 for GO and 0.955 for RGO, which further suggested that GO was reduced to RGO. The Raman spectrum of the CdS QDs/RGO composites also contained both D and G bands (at 1348 and 1590 cm−1, respectively), but the relative intensity of D/G increased up to 1.056, indicating the reduction of GO.57 Apart from this, some additional peaks appeared in the range of 100–1000 cm−1, indicating the good crystallinity of CdS.58
3.3 The photocatalytic properties of CdS QDs/RGO composites
The photocatalytic properties of pure CdS and CdS QDs/RGO composites with different GO contents were investigated, as shown in Fig. 11 and 12. All three kinds of CdS QDs/RGO composites showed better photocatalytic activity than pure CdS. The photocatalytic activity of the composites varied with different content of GO in the composites. The photodegradation rate of MB was 94.0% after irradiation for 40 min and reached 96.5% after irradiation for 140 min, which demonstrated that almost all the MB molecules in the solution were decomposed by composites (60). For pure CdS, however, the photodegradation rate of MB was only 64.8% after irradiation for 40 min and reached 90.6% after irradiation for 140 min. Moreover, based on our experiment, the photocatalytic performance was not getting better with increasing the content of GO. The photocatalytic performance of CdS QDs/RGO composites (60) was better than that of composites (30) and composites (90). This may be because the lower content of GO can not provide enough transport pathways for photo-generated electrons from excited CdS QDs, leading to the recombination of the electron–hole pairs. Likewise, the higher content of GO limited the diffusion of MB in the catalytically active site, shielded the incident light and thus reduced the light harvesting of CdS.59 | ||
| Fig. 11 Photodegradation of MB by different photocatalysts: (a) pure CdS, (b) CdS QDs/RGO composites (90), (c) CdS QDs/RGO composites (30), (d) CdS QDs/RGO composites (60). | ||
In order to reduce the impact of the physical adsorption on the photodegradation, we halved the amounts of photocatalysts. The photocatalytic properties of pure CdS and CdS QDs/RGO composites with different GO contents were investigated, as shown in Fig. 13. Only about 40% of MB was adsorbed for composites (60), and even fewer for other samples. All three kinds of CdS QDs/RGO composites showed better photocatalytic activity than pure CdS. The photodegradation rate of MB was 80.5% after irradiation for 40 min and reached 90.4% after irradiation for 140 min for composites (60). For pure CdS, however, the photodegradation rate of MB was only 54.1% after irradiation for 40 min and reached 82.9% after irradiation for 140 min.
 | ||
| Fig. 13 Photodegradation of MB by different photocatalysts (the amounts were halved): (a) pure CdS, (b) CdS QDs/RGO composites (90), (c) CdS QDs/RGO composites (30), (d) CdS QDs/RGO composites (60). | ||
3.4 The role of RGO in the composites during the photocatalytic reaction
In order to explore the role of RGO in the composites during the photocatalytic reaction, the transient photocurrent responses of CdS QDs/RGO composites and CdS were investigated under intermittent visible light illumination. As shown in Fig. 14, the photocurrent of CdS QDs/RGO composites was enhanced significantly compared to CdS, and the photocurrent rapidly decreased to zero as long as the light was switched off. This phenomenon indicated a more efficient separation of the photoexcited electron–hole pairs and longer lifetime of the photogenerated charge carriers. Moreover, no obvious photocurrent decay was observed, suggesting the effective transport of photogenerated electrons to RGO sheets.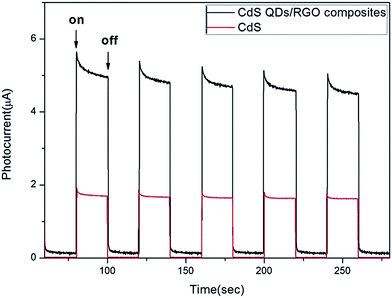 | ||
| Fig. 14 Transient photocurrent response of CdS QDs/RGO composites and CdS in 0.5 M Na2SO4 aqueous solution without bias versus Hg/Hg2Cl2 under the irradiation of visible light. | ||
Electrochemical impedance spectroscopy (EIS) is a very useful tool to characterize the charge-carrier migration. As shown in Fig. 15, the Nyquist plots of the CdS QDs/RGO composites and CdS under visible-light irradiation obtained in 0.5 M Na2SO4 electrolyte solution gave rise to semicycles at high frequencies. The charge transfer resistance can be directly measured as the semicircle diameter.60 CdS QDs/RGO composites showed smaller semicircles compared with pure CdS. The decreased semicircles indicated diminished resistance of working electrodes, suggesting a rapid transport of charge carriers and an effective charge separation after the introduction of RGO.61
 | ||
| Fig. 15 Electrochemical impedance spectroscopy (EIS) Nyquist plots of CdS QDs/RGO composites and CdS. | ||
Diffuse reflectance UV-visible spectra of pure CdS and CdS QDs/RGO composites in the wavelength range of 200–800 nm were obtained using a UV-vis scanning spectrophotometer (Hitachi U-3010), while BaSO4 was used as a reference. It can be seen from Fig. 16 that the pure CdS showed broad absorption band in the wavelength range of 200–500 nm. However, CdS QDs/RGO composites showed very broad absorption band covering the entire region. Like other carbon materials, RGO itself has good light absorption characteristics, and thus the range of available light wavelengths was extended after the introduction of RGO. Thereby, the light absorption of photocatalyst was improved, which might enhance the photocatalytic activities.
3.5 The possible photocatalytic mechanism of CdS QDs/RGO composites
Upon visible-light irradiation, the electron–hole pairs were generated on the surface of CdS. RGO was electrically conductive, and thus the photo-generated electrons in the conduction band of the CdS QDs migrated into RGO sheets easily. Consequently, the possibility of the recombination of the electron–hole pairs decreased, resulting in enhanced photocatalytic performance.62,63 The RGO sheets with free electrons could activate the dissolved oxygen to produce superoxide anion radical. Simultaneously, the left holes could react with H2O or OH− to form hydroxyl radical. The hydroxyl radical was a powerful oxidant and started a series of oxidation reactions, ultimately leading to total degradation of MB.64 The possible photodegradation mechanism of MB for CdS QDs/RGO composites is shown in Fig. 17. The improved photocatalytic activity could be mainly ascribed to the separation of photo-generated electron–hole pairs. RGO could serve as conductive optoelectronic scaffolds in composites, suppressing the recombination of photogenerated electron–hole pairs and the photo-corrosion of CdS. Also, the two dimensional sheet structure of RGO inhibited the aggregation of CdS QDs, which was beneficial to the absorption of the dye. The small size of CdS QDs in composites resulted in high photochemical stability of CdS and more active sites, enhancing the light harvesting.65–674. Conclusion
In summary, CdS QDs/RGO composites were prepared by a one-step vapor diffusion method at 80 °C for 12 h. The formation of CdS QDs and the reduction of GO were accomplished in a one-step reaction. Uniform CdS QDs with about 4–7 nm were well distributed on the RGO sheets. The contents of GO in composites had an effect on their photocatalytic performances. The CdS QDs/RGO composites (60) showed better photocatalytic activity than pure CdS, CdS QDs/RGO composites (30) and CdS QDs/RGO composites (90). The improved photocatalytic activity can be mainly ascribed to the separation of photo-generated electron–hole pairs.Acknowledgements
We thank the National Natural Science Foundation of China for financial support (grant no. 21376029 and 21071017).References
- Z. H. Han, H. Y. Zhu, J. Shi, G. Parkinson and G. Q. Lu, J. Solid State Chem., 2007, 180, 902–905 CrossRef CAS PubMed.
- Y. G. Sun, B. Mayers and Y. N. Xia, Nano Lett., 2003, 3, 675–677 CrossRef CAS.
- S. M. Wang, P. Liu, X. X. Wang and X. Z. Fu, Langmuir, 2005, 21, 11969–11976 CrossRef CAS PubMed.
- M. Bruchez, M. Moronne, P. Gin, S. Weiss and A. P. Alivisatos, Science, 1998, 281, 2013–2015 CrossRef CAS.
- J. Wang, G. Liu and I. A. Merkoc, J. Am. Chem. Soc., 2003, 125, 3214–3215 CrossRef CAS PubMed.
- J. Britt and C. Ferekides, Appl. Phys. Lett., 1993, 62, 2851–2852 CrossRef CAS PubMed.
- M. Tsuji, T. Aramoto, H. Ohyama, T. Hibino and K. Omura, J. Cryst. Growth, 2000, 214-215, 1142–1147 CrossRef.
- S. Chun, Y. Jung, J. Kim and D. Kim, J. Cryst. Growth, 2011, 326, 152–156 CrossRef CAS PubMed.
- M. R. Ma, L. Dai and G. G. Qin, Nano Lett., 2007, 7, 868–873 CrossRef PubMed.
- H. Li, J. M. Green and J. Jiao, J. Phys. Chem. C, 2008, 112, 15140–15143 CAS.
- X. Zong, J. Han, G. Ma, H. Yan, G. Wu and C. Li, J. Phys. Chem. C, 2011, 115, 12202–12208 CAS.
- X. Zong, H. Yan, G. Wu, G. Ma, F. Wen, L. Wang and C. Li, J. Am. Chem. Soc., 2008, 130, 7176–7177 CrossRef CAS PubMed.
- N. Bao, L. Shen, T. Takata and K. Domen, Chem. Mater., 2007, 20, 110–117 CrossRef.
- L. Jia, D. H. Wang, Y. X. Huang, A. W. Xu and H. Q. Yu, J. Phys. Chem. C, 2011, 115, 11466–11473 CAS.
- Q. Li, B. Guo, J. Yu, J. Ran, B. Zhang, H. J. Yan and J. R. Gong, J. Am. Chem. Soc., 2011, 133, 10878–10884 CrossRef CAS PubMed.
- L. Sheeney-Haj-Ichia, B. Basnar and I. Willner, Angew. Chem., Int. Ed., 2005, 44, 78–83 CrossRef CAS PubMed.
- M. H. Entezari and N. Ghows, Ultrason. Sonochem., 2011, 18, 127–134 CrossRef CAS PubMed.
- T. Dufaux, J. Boettcher, M. Burghard and K. Kern, Small, 2010, 6, 1868–1872 CrossRef CAS PubMed.
- S. C. Hayden, N. K. Allam and M. A. El-Sayed, J. Am. Chem. Soc., 2010, 132, 14406–14408 CrossRef CAS PubMed.
- P. Gao, J. Liu, T. Zhang, D. D. Sun and W. Ng, J. Hazard. Mater., 2012, 229–230, 209–216 CrossRef CAS PubMed.
- Z. Khan, M. Khannam, N. Vinothkumar, M. De and M. Qureshi, J. Mater. Chem., 2012, 22, 12090–12095 RSC.
- J. Cao, J. Z. Sun, J. Hong, H. Y. Li, H. Z. Chen and M. Wang, Adv. Mater., 2004, 16, 84–87 CrossRef CAS.
- Q. Huang and L. Gao, Nanotechnology, 2004, 15, 1855–1860 CrossRef CAS.
- N. Gaponik, D. V. Talapin, A. L. Rogach, K. Hoppe, E. V. Shevchenko, A. Kornowski, A. Eychmuller and H. Weller, J. Phys. Chem. B, 2002, 106, 7177 CrossRef CAS.
- M. Fu, Q. Z. Jiao and Y. Zhao, J. Mater. Chem. A, 2014, 2, 735–744 CAS.
- S. Stankovich, D. A. Dikin, G. H. B. Dommett, K. M. Kohlhaas, E. J. Zimney, E. A. Stach, R. D. Piner, S. T. Nguyen and R. S. Ruoff, Nature, 2006, 442, 282–286 CrossRef CAS PubMed.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- Z. He, G. Guai, J. Liu, C. Guo, J. S. Chye Loo, C. M. Li and T. T. Y. Tan, Nanoscale, 2011, 3, 4613–4616 RSC.
- J. Zhang, J. Yu, M. Jaroniec and J. R. Gong, Nano Lett., 2012, 12, 4584–4589 CrossRef CAS PubMed.
- L. F. Qi, J. G. Yu and M. Jaroniec, Phys. Chem. Chem. Phys., 2011, 13, 8915–8923 RSC.
- A. Cao, Z. Liu, S. Chu, M. Wu, Z. Ye, Z. Cai, Y. Chang, S. Wang, Q. Gong and Y. Liu, Adv. Mater., 2010, 22, 103–106 CrossRef CAS PubMed.
- Q. Li, B. Guo, J. Yu, J. Ran, B. Zhang, H. Yan and J. R. Gong, J. Am. Chem. Soc., 2011, 133, 10878–10884 CrossRef CAS PubMed.
- H. X. Chang, X. J. Lv, H. Zhang and J. H. Li, Electrochem. Commun., 2010, 12, 483–487 CrossRef CAS PubMed.
- W. S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS.
- J. J. Guo, S. M. Zhu, Z. X. Chen, Y. Li, Z. Y. Yu, Q. L. Liu, J. B. Li, C. L. Feng and D. Zhang, Ultrason. Sonochem., 2011, 18, 1082–1090 CrossRef CAS PubMed.
- P. C. Lian, X. F. Zhu, H. F. Xiang, Z. Li, W. S. Yang and H. H. Wang, Electrochim. Acta, 2010, 56, 834–840 CrossRef CAS PubMed.
- L. S. Zhang, L. Y. Jiang, H. J. Yan, W. D. Wang, W. Wang, W. G. Song, Y. G. Guo and L. J. Wan, J. Mater. Chem., 2010, 20, 5462–5467 RSC.
- Z. F. Du, X. M. Yin, M. Zhang, Q. Y. Hao, Y. G. Wang and T. H. Wang, Mater. Lett., 2010, 64, 2076–2079 CrossRef CAS PubMed.
- Z. Chen, S. Q. Liu, M. Q. Yang and Y. J. Xu, ACS Appl. Mater. Interfaces, 2013, 5, 4309–4319 CAS.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, M. I. Katsnelson, I. V. Grigorieva, S. V. Dubonos and A. A. Firsov, Nature, 2005, 438, 197–199 CrossRef CAS PubMed.
- P. Gao, J. C. Liu, D. D. Sun and W. Ng, J. Hazard. Mater., 2013, 250–251, 412–420 CrossRef CAS PubMed.
- M. Fu, Q. Z. Jiao and Y. Zhao, J. Mater. Chem. A, 2013, 1, 5577–5586 CAS.
- N. Zhang, Y. H. Zhang, X. Y. Pan, X. Z. Fu, S. Q. Liu and Y. J. Xu, J. Phys. Chem. C, 2011, 115, 23501–23511 CAS.
- Y. Q. Zhan, F. B. Meng, Y. J. Lei, R. Zhao, J. C. Zhong and X. B. Liu, Mater. Lett., 2011, 65, 1737–1740 CrossRef CAS PubMed.
- P. V. Kamat, J. Phys. Chem. Lett., 2010, 1, 520–527 CrossRef CAS.
- M. Fu, Q. Z. Jiao and Y. Zhao, J. Mater. Chem. A, 2014, 2, 735–744 CAS.
- S. Bai and X. P. Shen, RSC Adv., 2012, 2, 64–98 RSC.
- C. Xu, X. Wang and J. W. Zhu, J. Phys. Chem. C, 2008, 112, 19841 CAS.
- A. V. Murugan, T. Muraliganth and A. Manthiram, Chem. Mater., 2009, 21, 5004–5006 CrossRef CAS.
- H. K. Jeong, Y. P. Lee, R. J. Lahaye, M. H. Park, K. H. An, I. J. Kim, C. W. Yang, C. Y. Park, R. S. Ruoff and Y. H. Lee, J. Am. Chem. Soc., 2008, 130, 1362–1366 CrossRef CAS PubMed.
- C. Nethravathi, T. Nisha, N. Ravishankar, C. Shivakumara and M. Rajamathi, Carbon, 2009, 47, 2054–2059 CrossRef CAS PubMed.
- A. B. Bourlinos, D. Gournis, D. Petridis, T. Szabo, A. Szeri and I. Dekany, Langmuir, 2003, 19, 6050–6055 CrossRef CAS.
- G. Beamson and D. Briggs, High Resolution XPS of Organic Polymers: The Scienta ESCA300 Database, John Wiley and Sons, New York, 1992 Search PubMed.
- R. J. Waltman, J. Pacansky and C. W. Bates Jr, Chem. Mater., 1993, 5, 1799–1804 CrossRef CAS.
- D. Graf, F. Molitor, K. Ensslin, C. Stampfer, A. Jungen, C. Hierold and L. Wirtz, Nano Lett., 2007, 7, 238–242 CrossRef CAS PubMed.
- N. J. Bell, Y. H. Ng and A. J. Du, J. Phys. Chem. C, 2011, 115, 6004–6009 CAS.
- M. Fu, Q. Jiao and Y. Zhao, J. Mater. Chem. A, 2014, 2, 735–744 CAS.
- F. Tuinstra and J. L. Koenig, J. Chem. Phys., 1970, 53, 1126–1130 CrossRef CAS PubMed.
- T. Tsumura, N. Kojitani, I. Izumi, N. Iwashita, M. Toyoda and M. Inagaki, J. Mater. Chem., 2002, 12, 1391 RSC.
- D. Wang, D. Choi, J. Li, Z. Yang, Z. Nie, R. Kou, D. Hu, C. Wang, L. V. Saraf and J. Zhang, ACS Nano, 2009, 3, 907–914 CrossRef CAS PubMed.
- B. L. He, B. Dong and H. L. Li, Electrochem. Commun., 2007, 9, 425 CrossRef CAS PubMed.
- H. Zhang, X. J. Lv, Y. M. Li, Y. Wang and J. H. Li, ACS Nano, 2010, 4, 380 CrossRef CAS PubMed.
- Y. H. Zhang, Z. R. Tang, X. Z. Fu and Y. J. Xu, ACS Nano, 2010, 4, 7303 CrossRef CAS PubMed.
- S. A. Ali, J. R. Bolton and S. R. Cater, Sol. Energy, 1996, 56, 439–443 CrossRef.
- J. W. Tang, Z. G. Zou and J. H. Ye, Chem. Mater., 2004, 16, 1644 CrossRef CAS.
- J. C. Yu, X. C. Wang and X. Z. Fu, Chem. Mater., 2004, 16, 1523 CrossRef CAS.
- G. S. Li, D. Q. Zhang and J. C. Yu, Chem. Mater., 2008, 20, 3983 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |