Preparation of magnetic Pb(II) and Cd(II) ion-imprinted microspheres and their application in determining the Pb(II) and Cd(II) contents of environmental and food samples
Shoulian Wei*,
Yong Liu,
Mingdong Shao,
Ling Liu,
Hongwu Wang and
Yanqing Liu*
Faculty of Chemistry and Chemical Engineering, Zhaoqing University, Zhaoqing 526061, P.R. China. E-mail: weishlmary@126.com; yqliu@zqu.edu.cn
First published on 25th June 2014
Abstract
A novel magnetic ion-imprinted polymer (MIIP) was prepared by inverse microemulsion polymerization using ethylene glycol dimethacrylate (EGDMA) as the cross-linker, and 2,2-azo-bis-isobutyronitrile (AIBN) as the initiator. Fe3O4 particles were incorporated into the imprinted polymer matrix containing 4-vinyl pyridine and acrylate-modified Spirulina platensis as the functional monomers, Pb(II) and Cd(II) as double-templates. The MIIP product was characterized by Fourier-transform infrared spectroscopy, thermal gravimetric analysis and scanning electron microscopy and then evaluated for the magnetic solid-phase extraction of Pb(II) and Cd(II) from environmental and food samples. Inductively coupled plasma-optical emission spectrometry detection was also performed. The obtained MIIP showed the magnetic property, agglomerates of globular particles and rough surface morphologies. The maximum static adsorption capacities of MIIP for Pb(II) and Cd(II) were 108 and 56 mg g−1, respectively. The factors affecting the adsorption and separation of Pb(II) and Cd(II), namely, solution pH, adsorbent dosage, eluting solvent, and elution time, were investigated. Under the optimized conditions, the detection limits (S/N = 3) of the method were 0.1 and 0.08 μg kg−1 for Pb(II) and Cd(II), respectively. The relative standard deviations (RSDs) for 10-replicate detections of a soil sample were 3.3% and 3.7% for Pb(II) and Cd(II), respectively. Standard solutions containing Pb(II) and Cd(II) in the concentration range of 0.8 to 5000 μg kg−1 were analyzed by the proposed procedure and it was found that the calibration curve was linear in this range (R2 ≥ 0.999). The proposed method was successfully used to determine the Pb(II) and Cd(II) contents of cinnamon, lotus root and sweet potato with recovery rates ranging from 87.5% to 106%.
1. Introduction
Cadmium and lead are two of the most toxic elements to humans and animals, even at low concentrations.1 Lead causes chronic inflammation of the kidney and heart, inhibits brain development, and decreases nerve conduction velocity. Cadmium is carcinogenic to animals and humans, and chronic cadmium accumulation can lead to kidney damage, impaired regulation of calcium and phosphorus, bone demineralization, osteomalacia, and pathological fractures.2–4 Monitoring the level of lead and cadmium ions in environmental and food samples is gaining increased attention because these elements are a severe threat to animals, plants, humans, and the surrounding environment. Considering the low concentration of lead and cadmium ions in environmental and food samples, separation and pre-concentration are often necessary prior to the determination itself.Spirulina platensis (S. platensis) is a blue-green alga that is an alternative source of protein for human food and animal feeds.5 The surface of this alga has many –NH2, –COOH, –OH, and other charged groups that can serve as chelation sites for metals, and the organism can potentially act as a biosorbent for heavy metal removal.6–9 S. platensis has been used as well to remove Cd2+, Cr3+, Cu2+, Co2+, Zn2+, Pb2+, and Ni2+ ions from wastewater.10–12 S. platensis also shows great sorption capacity toward these metals, especially Pb2+, in both one- and three-metal systems. However, the organism lacks selectivity and cannot be rapidly and effectively separated after wastewater treatment.
Magnetic nanoparticles are attracting worldwide attention because of their excellent magnetic response, high dispersibility, relatively large surface area, and easy surface modification. Magnetite nanoparticles can reportedly remove Cr(VI), Pb2+, Hg2+ from wastewater and food samples.13–15 Li et al.16 reported the separation and accumulation of Cu(II), Zn(II), and Cr(VI) from aqueous solution using magnetic chitosan modified with diethylenetriamine. Sadeghi et al.17 reported the use of surface-modified magnetic Fe3O4 nanoparticles as a selective sorbent for the solid-phase extraction (SPE) of uranyl ions from water samples. However, all of these modified magnetic nanoparticles are applied in the non-specific absorption of metals and have low selectivity. Magnetic nanoparticles modified with ionic imprinted polymer have also been developed.18–24 Metal ion-imprinted polymers are synthesized with metal ion templates in the presence of a suitable monomer (vinylated reagent) or a monomer and an auxiliary/complexing non-vinylated reagent that can selectively distinguish the template ion from related analogous compounds. Different approaches to the synthesis of metal ion-imprinted polymers were reviewed by Rao et al.25 These conventional methods exhibit the drawbacks of low binding capacity and selectivity, slow mass-transfer rate, and few complexing ligands with vinyl groups. Latif et al.26 reported the synthesis of Ni2+ and Cu2+ imprinted polymer by functionalized sol–gel materials containing amino group as ligand.
The hierarchical imprinting method was introduced by Dai et al.27 with surfactant micelles and metal ions as template. Hierarchically imprinted polymers can be precisely controlled at adsorption sites and pore structures. Dickert et al.28 introduced the idea of “double molecular imprinting” using anthracene and chrysene as templates that were simultaneously imprinted in a crosslinked polyurethane thin film, which was able to recognize both the templates. Compared with single-template imprinted polymers, hierarchically imprinted polymers and double-template imprinted polymers demonstrate high binding capacity, high selectivity, and fast mass transfer.29,30 The use of vinylated S. platensis instead of non-vinylated S. platensis to fix the complexing agent to the polymeric matrix also reportedly improves selectivity toward heavy metals.31
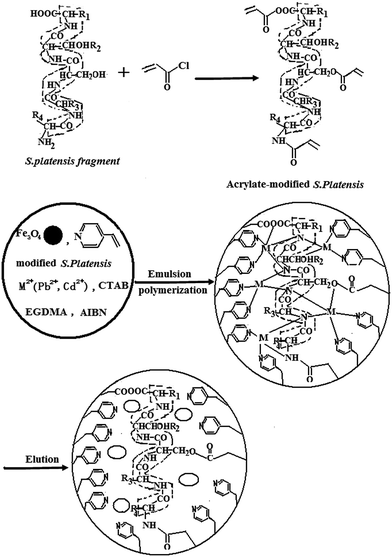
In the present study, a novel magnetic ion-imprinted polymer (MIIP) was synthesized by water-in-oil-in-water (W/O/W) emulsions polymerization using lead and cadmium ions as double-templates, 4-vinylpyridine (4-VP) and acrylate-modified S. platensis as functional monomer with Fe3O4 particles existing. W/O/W emulsions are three-phase systems in which small internal aqueous droplets, surrounded by a primary surfactant-stabilizing layer, are dispersed in the oil phase, which, in turn, is dispersed in the external aqueous phase and is also surrounded by a secondary surfactant layer. After preparing a stable W/O/W emulsion composed of water1, monomers, and water2, adding initiator, a radical polymerization was carried out selectively in the monomer oil phase. The resulting polymer (MIIP) was separated by a magnet and was washed several times with 0.1 mol L−1 EDTA solution and with methanol several times to remove lead and cadmium ions, organic solvent, internal aqueous droplets and surfactant micelles etc., respectively. The removal of the metal ion from the polymer leaves cavities that exhibit ionic recognition. The removal of the organic solvent and internal aqueous droplets will probably result in the formation of rough and porous structures or multihollow structures which includes large surface areas and excellent metal ion transport kinetics.32 The MIIP was characterized by Fourier-transform infrared spectroscopy (FTIR), Thermal gravimetric analysis (TGA) and scanning electron microscopy (SEM). The adsorption performance, reusability, and application of MIIP for Pb(II) and Cd(II) were investigated in detail.
2. Materials and methods
2.1. Materials and instruments
Cd(NO3)2, Pb(NO3)2, Mg(NO3)2, Zn(NO3)2, and Cu(NO3)2 standards were supplied by CMP Sinoexpo Biological Technology Co., Ltd. (Shanghai, China). Tween 80, Span 80, ferrous chloride (FeCl2·4H2O), and ferric chloride (FeCl3·6H2O) were obtained from Tianjin Chemical Reagent Co. (Tianjin, China). Carboxymethyl cellulose (CMC) and cetyltrimethylammonium bromide (CTAB) were purchased from Pure Crystal Shanghai Reagents Co., Ltd. (Shanghai, China). Azodiisobutyronitrile (AIBN), 4-VP, and ethylene glycol dimethacrylate (EGDMA) were purchased from Aladdin Chemistry Co., Ltd. (Shanghai, China). S. platensis was provided by the Zhaoqing Bangjian Pharmacy Plant in China. Water was purified through a Milli-Q water system (Bedford, USA). All other chemicals were analytical-reagent grade.UV-vis spectra and absorbance were measured using a double beam spectrophotometer (GBC 916 UV-vis model). FTIR spectra were recorded with a Shimadzu model FTIR-84003 spectrophotometer. The surface morphology and structure of the materials were identified using a SEM system (NGB4-DXS-10AC). The specific surface areas and average pore size of the polymers were measured from nitrogen adsorption data according to the Barrett–Joyner–Halenda (BJH) and Brunauer–Emmett–Teller (BET) methods, respectively, using an ASAP2020M + C system (Micromeritics, USA). A Thermo Electron inductively coupled plasma atomic emission spectrometer (ICAP-6300-DUO, Thermo Electron, USA) was used for multi-elemental determinations. pH was measured using a PHS-3C pH meter (Dapu Instrumentation Corp., Ltd. Shanghai, China). Thermal gravimetric analysis was carried out on a NETZSCH STA 449F3 instrument under N2 atmosphere at the heating rate of 10 °C min−1.
2.2. Preparation of Fe3O4 particles
Fe3O4 magnetic nanoparticles were prepared as previously reported33 with modifications. FeCl3·6H2O (1.35 g) and FeCl2·4H2O (0.6 g) were dissolved in 16 mL of deionized water under magnetic stirring at 40 °C and nitrogen bubbling. Then, 1.6 mL of 25% ammonia solution was added. The resulting suspension was stirred and heated at 50 °C for 1 h. The suspension was decanted, and the black residue was washed several times with deionized water to neutralize the aqueous solution. Three grams of emulsifier (mSpan 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mTween 80 = 1
mTween 80 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) was added, and the mixture was stirred and heated at 80 °C for 1 h. The resulting material was washed four times with ethanol and dried in a vacuum.
2) was added, and the mixture was stirred and heated at 80 °C for 1 h. The resulting material was washed four times with ethanol and dried in a vacuum.
2.3. Acrylate-modified S. platensis
A 250 mL three-necked flask equipped with a condenser and an anhydrous CaCl2 drying tube, thermometer, and hydrochloric acid (HCl) tail gas absorption device was placed in a heat collection-type oil bath under magnetic stirring. Acrylic (1.2 mol) and SOCl2 (1.0 mol) were added to the flask, stirred, and heated at 40 °C for 2 h. Acrylic acid chloride was collected after distilling the mixture to remove the solvent.A 250 mL three-necked flask equipped with a dropping funnel and thermometer was placed in the heat collection-type oil bath under magnetic stirring. 4.0 g dry S. platensis powder, 5 mL of triethylamine, and 40 mL acetone were added to the flask under magnetic stirring and nitrogen bubbling, slowly dripping a mixture solution of the collected acryloyl chloride and 25.0 mL acetone. The resulting solution was stirred in an ice-cooled bath for 8 h and at room temperature for 12 h. The reaction solution was transferred to a rotary evaporator from which vacuum distillation was performed to remove the acetone. The resulting material was filtered and acrylate-modified S. platensis was obtained. A schematic diagram of this synthesis was presented in Fig. 1.
2.4. Preparation of lead and cadmium ion-imprinted microspheres
The imprinted polymer, lead, and cadmium ion-imprinted microspheres were synthesized by emulsion polymerization. In a typical procedure, 3.0 g of emulsifier (Span 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Tween 80 = 1
Tween 80 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) was dissolved in a mixture of 20 mmol EGDMA and 10 mL toluene under magnetic stirring. A 20 mL aqueous solution containing 3.0 g of acrylate-modified S. platensis, 3.0 mmol 4-VP, 1.0 mmol Pb(NO3)2, 1.0 mmol Cd(NO3)2, 1.5 g Fe3O4 particles, and 0.5 g CMC was added to the above solution. The mixture was sonicated for 20 min to yield W/O emulsions. The emulsions were placed in a 150 mL aqueous solution containing 0.15 g CTAB, which was stirred at rate of 400 r per min to form a W/O/W emulsion. The oxygen in the emulsion was removed by bubbling nitrogen for 10 min. After the addition of 0.2 g AIBN, the mixture was polymerized at 70 °C for 12 h under nitrogen flow and stirred at a rate of 400 r per min. The resulting polymer (MIIP) was placed in a 150 mL Erlenmeyer flask and separated by a magnet, then the polymer was washed with 0.1 mol L−1 EDTA solution several times (5 min each times) under ultrasound until no lead and cadmium ions were detectable in the effluent, after which the polymer was washed with methanol several times to remove surfactant micelle etc. and dried under vacuum.
3) was dissolved in a mixture of 20 mmol EGDMA and 10 mL toluene under magnetic stirring. A 20 mL aqueous solution containing 3.0 g of acrylate-modified S. platensis, 3.0 mmol 4-VP, 1.0 mmol Pb(NO3)2, 1.0 mmol Cd(NO3)2, 1.5 g Fe3O4 particles, and 0.5 g CMC was added to the above solution. The mixture was sonicated for 20 min to yield W/O emulsions. The emulsions were placed in a 150 mL aqueous solution containing 0.15 g CTAB, which was stirred at rate of 400 r per min to form a W/O/W emulsion. The oxygen in the emulsion was removed by bubbling nitrogen for 10 min. After the addition of 0.2 g AIBN, the mixture was polymerized at 70 °C for 12 h under nitrogen flow and stirred at a rate of 400 r per min. The resulting polymer (MIIP) was placed in a 150 mL Erlenmeyer flask and separated by a magnet, then the polymer was washed with 0.1 mol L−1 EDTA solution several times (5 min each times) under ultrasound until no lead and cadmium ions were detectable in the effluent, after which the polymer was washed with methanol several times to remove surfactant micelle etc. and dried under vacuum.
The single magnetic ion-imprinted polymer (MIIP/Pb and MIIP/Cd) was prepared by following the procedure mentioned above using Pb(NO3)2 and Cd(NO3)2 as a template, respectively. Magnetic non-imprinted polymer (MNIP) was also prepared by the same procedure but without the addition of Pb(NO3)2 and Cd(NO3)2 (Fig. 1).
2.5. Adsorption experiments
Adsorption experiments were performed by batch method in aqueous solution. MIIP or MNIP of 30.0 mg weight was added to 10 mL sodium acetate–acetic acid solutions containing Pb(II) and Cd(II) with initial concentrations of 0.20 mg mL−1 to 1.20 mg mL−1 (ref. 19) in 150 mL conical flasks, then shaken at 150 rpm for 2 h at room temperature. MIIP was collected with the help of an external magnetic force. The supernatant was analyzed by inductively coupled plasma-optical emission spectrometry (ICP-OES), the adsorption amount of Pb(II) and Cd(II) was calculated using eqn (1), and the binding isotherm was plotted.
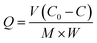 | (1) |
The binding isotherm of MNIP for Pb2+ or Cd2+ ions was measured using similar procedures as with MIIP for Pb2+ or Cd2+ ions.
All the experiments were performed in duplicates and the average values were recorded.
2.6. Selectivity experiments
The competitive absorption experiments were conducted with Cu2+ and Zn2+ ions as the structural analogues of the template Cd2+ and Pb2+ ions, and with Mg2+ as the reference metal ion. The experiment was carried out by adding 30.0 mg of sorbents (MIIP, MNIP, MIIP/Pb and MIIP/Cd) in an Erlenmeyer flask with 25.0 mL standard solution of each metal ion at an initial concentration of 1.0 × 10−3 mol L−1 and pH 6. The solution was shaken and adsorbed for 30 min at room temperature, and the supernatant was analyzed by ICP-OES. The distribution coefficient and selection coefficient were calculated by the following formula:
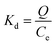 | (2) |
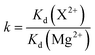 | (3) |
The relative selectivity coefficient k′ used to estimate the effect of imprinting on selectivity is defined by eqn (4):34
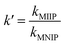 | (4) |
2.7. Sample preparation
A soil sample was collected in a plastic bag from an industrial zone in Dinghu Zhaoqing, China, transported to the laboratory as fast as possible, air-dried, pulverized, and sieved through a 0.1 mm mesh. Cinnamon, lotus root, and sweet potato samples were acquired from local supermarkets, transported to the laboratory, dried, and ground. The dried soil, cinnamon, lotus root, or sweet potato sample (2.0000 g) was weighed into a Teflon flask and decomposed with 10.0 mL of concentrated HNO3 and 1.0 mL of 30% (v/v) H2O2. The mixture was heated at 100 °C and evaporated nearly to dryness. After cooling, the sample was diluted to 50 mL with a sodium acetate–acetic acid buffer solution of pH 6.0.3. Results and discussion
3.1. Characterization of MIIP and MNIP
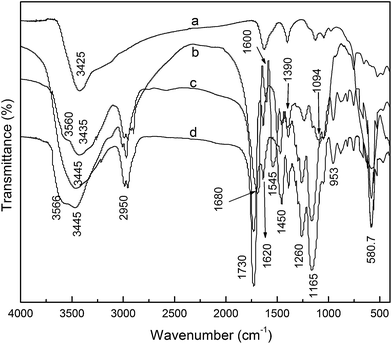
The FTIR spectra of Fe3O4, acrylate-modified S. platensis, MIIP and MNIP were shown in Fig. 2. A characteristic Fe–O band at about 580 cm−1 was found in MIIP and MNIP spectra, which indicated that Fe3O4 was embedded in polymers. The characteristic peaks of –NH or –OH, –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, –C
O, –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, –CO–NH and –C–O groups were at 3445 cm−1, 1680 cm−1, 1600 cm−1, 1545 1260 cm−1 and 1094 cm−1 in acrylate-modified S. platensis, respectively. In MIIP and MNIP spectra, the wide and strong adsorption bands at about 3566 and 3450 cm−1 were due to the stretching vibrations of –NH and –OH groups from S. platensis, respectively. The stretching bands and bending vibration bands of CH3 were observed at about 2950 cm−1, 1450 cm−1 and 1390 cm−1, respectively. The strong absorption bands at 1730 cm−1 indicated the existence of a large number of carbonyl functional groups (–COO) derived from EGDMA and S. platensis. Compared to the peak at 1600 cm−1 of acrylate-modified S. platensis, MIIP and MNIP have very weak C
C, –CO–NH and –C–O groups were at 3445 cm−1, 1680 cm−1, 1600 cm−1, 1545 1260 cm−1 and 1094 cm−1 in acrylate-modified S. platensis, respectively. In MIIP and MNIP spectra, the wide and strong adsorption bands at about 3566 and 3450 cm−1 were due to the stretching vibrations of –NH and –OH groups from S. platensis, respectively. The stretching bands and bending vibration bands of CH3 were observed at about 2950 cm−1, 1450 cm−1 and 1390 cm−1, respectively. The strong absorption bands at 1730 cm−1 indicated the existence of a large number of carbonyl functional groups (–COO) derived from EGDMA and S. platensis. Compared to the peak at 1600 cm−1 of acrylate-modified S. platensis, MIIP and MNIP have very weak C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C vibrations at 1620 cm−1, suggesting that the C
C vibrations at 1620 cm−1, suggesting that the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond is broken up after polymerization. The band at 1450 cm−1 was attributed to the bending vibrations of C–N from 4-VP19 and S. platensis. The strong adsorption bands at 1260 and 1165 cm−1 indicated the existence of C–O stretching vibration in polymers. In comparison with the infrared data of acrylate-modified S. platensis and Fe3O4, the existence of these characteristic peaks in MIIP and MNIP showed the successful coating of the magnetic core by a polymeric layer, which was synthesized using acrylate-modified S. platensis and 4-VP.
C double bond is broken up after polymerization. The band at 1450 cm−1 was attributed to the bending vibrations of C–N from 4-VP19 and S. platensis. The strong adsorption bands at 1260 and 1165 cm−1 indicated the existence of C–O stretching vibration in polymers. In comparison with the infrared data of acrylate-modified S. platensis and Fe3O4, the existence of these characteristic peaks in MIIP and MNIP showed the successful coating of the magnetic core by a polymeric layer, which was synthesized using acrylate-modified S. platensis and 4-VP.
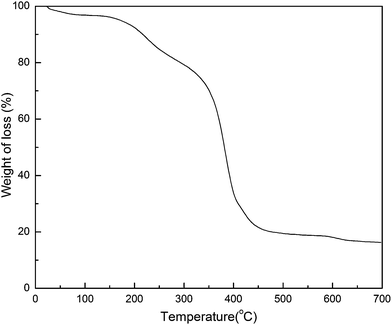
For the determination of the amount of coated polymer and Fe3O4 encapsulated in MIIP, the TG analysis was performed on the MIIP. As shown in Fig. 3, as the temperature grew to 143 °C, the mass of MIIP lost 3.32%, which probably caused by the evaporation of water molecules. From 143 to 309 °C, the thermal degradation of MIIP lost 18.12% of mass, which might be due to the decomposition free small molecular in MIIP. When the temperature rose to 572 °C, a significant mass loss of 60.36% was observed, mainly caused by the degradation of polymer. The final residue (16.23%) at 700 °C was found to be consistent with calculated value for 1.5 g of Fe3O4 particles in the MIIP (calc. 16.65%). Thus, this magnetic MIP was stable up to 300 °C and about 62% and 16% of this composite was polymer and Fe3O4, respectively.
SEM images of the morphological features of Fe3O4 (M), MIIP (A) and MNIP (B) are shown in Fig. 4. The particle size of Fe3O4, MIIP and MNIP is about 60 nm, 500 nm and 450 nm, respectively. The MIIP and MNIP particles exhibited similar agglomerates of globular particles and rough surface. The specific surface area, average pore diameter and pore volume of Fe3O4, MIIP and MNIP were summarized in Table 1. As shown in Table 1, the surface areas, average pore diameter of MIIP and MNIP were higher than the Fe3O4. There were no significant differences in specific surface areas, average pore diameter, and pore volume between MIIP and MNIP. Therefore, the different adsorption capacity between the MIIP and MNIP in the study could be due to imprinting effect and not due to the morphological difference.
| Sample | The specific surface area (m2 g−1) | Average pore diameter (nm) | Pore volumes (m3 g−1) |
|---|---|---|---|
| Fe3O4 | 113 | 1.8 | 0.211 |
| MIP | 261.5 | 3.4 | 0.293 |
| NIP | 284.6 | 3.6 | 0.276 |
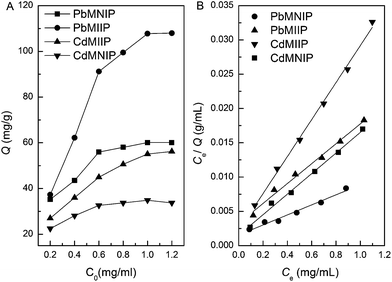
3.2. Adsorption behavior of magnetic imprinted polymers
The Langmuir equation was also used to evaluate the behavior of adsorbent:30
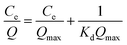 | (5) |
| Metal ion | Q (mg g−1) | Kd (L g−1) | k | k′ | |||
|---|---|---|---|---|---|---|---|
| MIIP | MNIP | MIIP | MNIP | MIIP | MNIP | ||
| Pb2+ | 108 | 55.9 | 1.38 | 0.40 | 2.94 | 1.11 | 2.65 |
| Cd2+ | 56.2 | 31.5 | 1.25 | 0.42 | 2.66 | 1.17 | 2.27 |
| Cu2+ | 26.0 | 17.1 | 0.81 | 0.40 | 1.72 | 1.11 | 1.55 |
| Zn2+ | 24.9 | 17.0 | 0.70 | 0.38 | 1.49 | 1.06 | 1.41 |
| Mg2+ | 7.29 | 6.10 | 0.47 | 0.36 | |||
3.3. Desorption and repeated use
To investigate the reusability of the MIIP sorbents, the adsorption–desorption cycle was checked 10 times using the same MIIP sorbents and with 0.1 mol L−1 EDTA as the eluting solvent. The results shows that the adsorption capacity of MIIP for Pb(II) and Cd(II) ions slowly decreased with increased cycle number. After seven cycles, the adsorption capacity of MIIP for Pb(II) and Cd(II) ions decreased by 9.6% and 11.9%, respectively, indicating that MIIP had good reusability and stability for Pb(II) and Cd(II) adsorption.3.4. Optimization of magnetic SPE (M-SPE) conditions
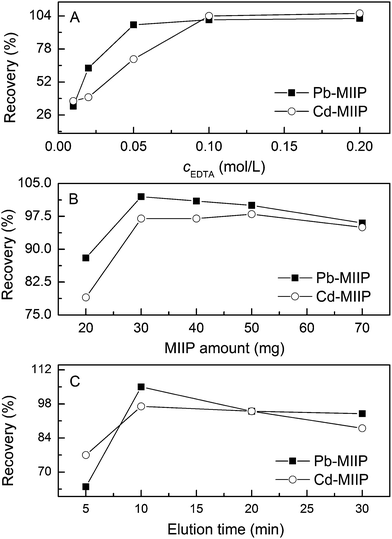
The type and concentration of eluting agent, MIIPs amount, extraction time, elution time and sample volume affect the recovery of the extraction. These parameters were investigated by analyzing spiked samples (1.0 μg mL−1) in order to get the optimum extracting conditions.Elution experiments were performed with 0.1 mol L−1 HCl, 0.1 mol L−1 HNO3, and 0.1 mol L−1 EDTA. Among these solutions, 0.1 mol L−1 of EDTA solution was found to be most effective for desorbing Pb(II) and Cd(II) ions from the loaded adsorbents. The reason is that the complexing action between the EDTA with Pb(II) and Cd(II) is in favor of the desorption of Pb(II) and Cd(II) ions from the MIIP. To achieve maximum recovery of Pb(II) and Cd(II) ions, the effect of different concentrations of EDTA, ranging from 0.01 mol L−1 to 0.2 mol L−1, on Pb(II) and Cd(II) recovery was investigated by ultrasound-aided elution. The results in Fig. 6A show that the recovery increased dramatically with increased EDTA concentration up to 0.10 mol L−1, beyond which the recovery remained constant. Thus, an EDTA concentration of 0.10 mol L−1 was selected for the succeeding experiments.
Different mounts of MIIP sorbent ranging from 20 mg to 70 mg were applied to 30 mL spiked samples. The results presented in Fig. 6B demonstrate that a high recovery ranging from 102% to 97% was obtained at an MIIP amount range of 30 mg to 50 mg. Further increase in the amount of sorbent led to a decrease in recovery. Thus, 50 mg of the sorbent was chosen as the optimum amount.
The extraction and elution time is a key factor in the M-SPE procedure. The extraction and elution time ranging from 0 min to 30 min was studied. The results showed that 2 min for a completing the extraction was achieved. For the elution time, as shown in Fig. 6C, the recovery increased as the elution time increased from 0 min to 10 min, then slightly declined from 10 min to 30 min, possibly because the Pb(II) and Cd(II) ions in the solution rebound to the sorbent. Thus, the optimum extraction and elution time was set at 2 min and 10 min, respectively.
The optimization experiment of sample volume was performed with 10–200 mL spiked samples. It was found that high recoveries were obtained when sample volume was equal or less than 100 mL. Therefore, 100 mL was selected as the break-through volume.
3.5. Effect of coexisting ions
The effect of various possible coexisting ions, such as SO42−, CO32−, NO3−, PO43−, NH4+, K+, Na+, Ca2+, Fe3+, Co2+, Mn2+, Ni2+, and Cr3+ was investigated. The tolerance limit of the coexisting ions was defined as the maximum concentration of foreign substances that can cause approximately ±5% relative error in determination. The following ion concentrations were found not to affect the absorption capacity: 1000-fold NO3−, NH4+, K+, and Na+; 500-fold Ca2+ and Fe3+; 200-fold Mn2+, Cr3+, SO42−, CO32−, and PO43−; and 100-fold Co2+ and Ni2+ ions, which imply that MIIP exhibits high selectivity for Pb(II) and Cd(II) in the presence of other metal ions. The main possible reasons for the high selectivity are that the recognition sites of the double template Pb(II) and Cd(II) imprinted sorbent for the chelating amine group and hydroxyl group are arranged in a suitable way for coordination-geometry selectivity, and that the hole size selectivity is dominated by Pb(II) and Cd(II) ions.30,36 Anion, which strongly bind with Pb(II) and Cd(II) ions, can influence the adsorption of Pb(II) and Cd(II) ions. The order of effect is PO43− > CO32− > SO42−. Therefore, the sample can't use sulfuric acid and phosphoric acid to dissolve.3.6. Validation and applications
A method based on M-SPE coupled to ICP-OES was established. Under the optimum conditions, an external aqueous calibrations in 0.1% nitric acid and standard addition calibrations in blank samples extract were constructed containing Pb(II) and Cd(II) ions between 0.8 to 5000 μg kg−1 in order to study possible matrix effects. The calibration equation in aqueous was y = 0.00387 + 0.9863 cPb2+ (R2 = 0.9998) and y = 0.00187 + 0.9983 cCd2+ (R2 = 1) for Pb(II) and Cd(II), respectively. The calibration equation in matrix was y = 0.00202 + 0.9605 cPb2+ (R2 = 0.999) and y = 0.00096 + 0.9718 cCd2+ (R2 = 0.9992) for Pb(II) and Cd(II), respectively. There are no significant differences of the calibration curve slopes for Pb or Cd obtained both for in aqueous and for in matrix. The results showed that the matrix could be efficiently removed during the M-SPE pre-concentration stage. Therefore, the external aqueous calibration in 0.1% nitric acid can be used to determine Pb and Cd in practical samples after M-SPE pre-concentration procedure.Limit of detection (LOD) and limit of quantification (LOQ) were calculated according to LOD = (3SD)/m and LOQ = (10SD)/m, where SD is the standard deviation of 10-replicate measurements of a procedural blank (acidified Milli-Q water treated as a sample) and m is the slope of the external aqueous calibration curve. The LODs were found to be 0.10 and 0.08 μg kg−1 for the Pb(II) and Cd(II) ions, respectively. Similarly, the LOQs were found to be 0.34 and 0.27 μg kg−1 for the Pb(II) and Cd(II) ions, respectively. These LODs and LOQs values are similar to those reported by other authors when using M-SPE combined with ICP-OES (0.18 μg L−1 for Pb37) or FAAS (0.11 μg L−1 for Cd38) detection. However, that ICP-MS will give a higher sensitivity by a factor of 100–1000 (0.004 μg L−1 for Pb using ionic imprinted polymer based solid phase extraction).37
The precision of the overall procedure was assessed by analyzing a contaminative soil (Section 2.7) 10 replicate. The determined mean values were 3.38 ± 0.11 and 1.08 ± 0.04 mg kg−1 for Pb and Cd, respectively. The relative standard deviation (RSD) was 3.3% and 3.7% for Pb and Cd, respectively, indicating that the method had good precision. The accuracy of the method was evaluated by recovery tests at three spiked levels (2.0, 5.0, and 10.0 mg kg−1) and by analysis of certified reference materials. The recoveries obtained from soil were 96.5 ± 3.3%, 95.4 ± 3.3% and 92.5 ± 3.3% for 2.0, 5.0, and 10.0 mg kg−1 of Pb, and 98.8 ± 3.7%, 97.1 ± 3.7%, 94.8 ± 3.7% for 2.0, 5.0, and 10.0 mg kg−1 of Cd, respectively, indicating that the developed method is reliable for determining Pb and Cd in environmental samples. Furthermore, GBW07402 soil certified reference materials were determined by duplicate. The determined mean values were 19.2 ± 0.7 and 0.068 ± 0.004 mg kg−1 for Pb and Cd, respectively, which were coincident with the corresponding certified values 20.0 ± 3.0 and 0.071 ± 0.014 mg kg−1 for Pb and Cd, respectively.
To further demonstrate the application of the method, cinnamon, lotus root and sweet potato samples (Section 2.7) were detected. The results are listed in Table 3, which shows that the recovery rates of Pb and Cd were in the range of 87.5% to 103% and 89.0% to 106%, respectively, indicating that the M-SPE procedure has a high selectivity for extracting Pb(II) and Cd(II) from food samples.
| Sample | Analyte | Found (mg kg−1) | Spiked (ng g−1) | Sum | Recovery (%) |
|---|---|---|---|---|---|
| Lotus root | Pb | 2.28 ± 0.08 | 10.0 | 11.40 ± 0.55 | 91.2 |
| 25.0 | 23.58 ± 0.50 | 85.2 | |||
| 50.0 | 47.98 ± 0.58 | 91.4 | |||
| Cd | 0.18 ± 0.05 | 8.0 | 8.18 ± 0.42 | 100 | |
| 20.0 | 18.22 ± 0.47 | 90.2 | |||
| 40.0 | 36.10 ± 1.12 | 89.8 | |||
| Sweet potato | Pb | 0.74 ± 0.02 | 10.0 | 10.84 ± 0.16 | 101 |
| 25.0 | 23.04 ± 1.13 | 89.2 | |||
| 50.0 | 47.09 ± 1.04 | 92.7 | |||
| Cd | 0.06 ± 0.00 | 8.0 | 7.87 ± 0.27 | 97.6 | |
| 20.0 | 17.88 ± 0.45 | 89.1 | |||
| 40.0 | 35.18 ± 1.47 | 87.8 | |||
| Cinnamon (2-3899) | Pb | 7.07 ± 0.24 | 10.0 | 15.96 ± 0.56 | 88.9 |
| 25.0 | 29.70 ± 1.19 | 90.5 | |||
| 50.0 | 49.27 ± 1.13 | 84.4 | |||
| Cd | 0.60 ± 0.02 | 8.0 | 8.60 ± 0.15 | 100 | |
| 20.0 | 21.80 ± 1.30 | 106 | |||
| 40.0 | 38.88 ± 0.82 | 95.7 | |||
| Commercial cinnamon | Pb | 12.3 ± 0.40 | 10.0 | 21.53 ± 0.50 | 92.3 |
| 25.0 | 33.70 ± 1.15 | 85.6 | |||
| 50.0 | 56.05 ± 1.50 | 87.5 | |||
| Cd | 0.24 ± 0.00 | 8.0 | 8.24 ± 0.27 | 100 | |
| 20.0 | 21.24 ± 0.34 | 105 | |||
| 40.0 | 39.16 ± 0.58 | 97.3 | |||
| Contaminated soil | Pb | 3.38 ± 0.11 | 10.0 | 12.03 ± 0.32 | 86.5 |
| 25.0 | 26.23 ± 1.42 | 91.4 | |||
| 50.0 | 48.63 ± 1.02 | 90.5 | |||
| Cd | 1.08 ± 0.03 | 8.0 | 9.08 ± 0.20 | 100 | |
| 20.0 | 20.10 ± 0.63 | 95.1 | |||
| 40.0 | 38.20 ± 1.25 | 92.8 |
The determined Pb(II) values in the lotus root, sweet potato, cinnamon (2-3899) and commercial cinnamon samples, which were 2.28 ± 0.08, 0.74 ± 0.03, 7.07 ± 0.25 and 12.30 ± 0.42 mg kg−1, respectively, were much higher than Chinese standard (Pb ≤ 0.2 mg kg−1 for potato crops and cinnamon). The determined Cd(II) values in the lotus root and sweet potato samples, which were 0.18 ± 0.01, and 0.06 ± 0.00 mg kg−1, respectively, were lower than the Chinese standard (Cd ≤ 0.2 mg kg−1 for potato crops). The determined Cd(II) values in the cinnamon (2-3899) and commercial cinnamon samples, which were 0.60 ± 0.02 and 0.24 ± 0.01 mg kg−1, respectively, were higher than the Chinese standard (Cd ≤ 0.2 mg kg−1 for cinnamon).
4. Conclusions
In this paper, a novel Pb–Cd–MIIP with double template and two functional monomers (4-VP and acrylate-modified S. platensis) were successfully synthesized by inverse microemulsion polymerization method. The sorbent exhibited high selectivity, large adsorption capacity, fast adsorption rate, and good reusability and stability for Pb(II) and Cd(II) ions. Pb–Cd–MIIP was successfully used with M-SPE materials for the rapid separation and enrichment of Pb(II) and Cd(II) in food and environmental samples, avoiding the time-consuming column passing and filtration steps in traditional SPE. The precision and accuracy of the presented method were satisfactory. The proposed method provided a rapid and effective tool for the extraction of trace amounts of Pb(II) and Cd(II) in all kinds of samples.Acknowledgements
This work is financially supported by the Science and Technology Innovation Project of Guangdong provincial education department (no. 2012kjcx0104), the Science and Technology Planning Project of Guangdong province (no. 2011B020314011) and the Natural Science Foundation of Guangdong Province (no. S2011010004004; no. S2012040007710).References
- G. F. Nordberg, B. A. Fowler, M. Nordberg and L. T. Friberg, Handbook on the Toxicology of Metals, Academic Press Inc., 2007 Search PubMed.
- H. Needleman, Lead Poisoning, Annu. Rev. Med., 2004, 55, 209 CrossRef CAS PubMed.
- B. Hultberg, A. Andersson and A. Isaksson, Toxicology, 1998, 126, 203 CrossRef CAS.
- M. P. Waalkes and S. Rehm, Fundam. Appl. Toxicol., 1992, 19, 512 CrossRef CAS.
- O. Ciferri, Spirulina, Microbiol. Rev., 1983, 47, 551 CAS.
- J. Wang and C. Chen, Biotechnol. Adv., 2009, 27, 195 CrossRef CAS PubMed.
- V. K. Gupta, A. Rastogi and A. Nayak, J. Colloid Interface Sci., 2010, 342, 533 CrossRef CAS PubMed.
- A. Çelekli, M. Yavuzatmaca and H. Bozkurt, J. Hazard. Mater., 2010, 173, 123 CrossRef PubMed.
- L. Fang, C. Zhou, P. Cai, W. Chen, X. Rong, K. Dai, W. Liang, J. Gu and Q. Huang, J. Hazard. Mater., 2011, 190, 810 CrossRef CAS PubMed.
- A. Seker, T. Shahwan, A. E. Eroglu, S. Yılmaz, Z. Demirel and M. C. Dalay, J. Hazard. Mater., 2008, 154, 973 CrossRef CAS PubMed.
- N. Rangsayatorn, P. Pokethitiyook, E. S. Upatham and G. R. Lanza, Environ. Int., 2004, 30, 57 CrossRef CAS.
- S. V. Gokhale, K. K. Jyoti and S. S. Lele, Bioresour. Technol., 2008, 99, 3600 CrossRef CAS PubMed.
- J. Hu, M. C. Lo and G. H. Chen, Water Sci. Technol., 2004, 50, 139 CAS.
- K. Cheng, Y. M. Zhou, Z. Y. Sun, H. B. Hu, H. Zhong, X. K. Konga and Q. W. Chen, Dalton Trans., 2012, 41, 5854 RSC.
- M. K. Rofouei, A. Rezaei, M. Masteri-Farahania and H. Khanib, Anal. Methods, 2012, 4, 959 RSC.
- H. Li, S. Bi, L. Liu, W. Dong and X. Wang, Desalination, 2011, 278, 397 CrossRef CAS PubMed.
- S. Sadeghi, H. Azhdari, H. Arabi and A. Z. Moghaddam, J. Hazard. Mater., 2012, 215–216, 208 CrossRef CAS PubMed.
- L. Chen, X. Zhang, L. Sun, Y. Xu, Q. Zeng, H. Wang, H. Xu, A. Yu, H. Zhang and L. Ding, J. Agric. Food Chem., 2009, 57, 10073 CrossRef CAS PubMed.
- Y. Ji, J. Yin, Z. Xu, C. Zhao, H. Huang, H. Zhang and C. Wang, Anal. Bioanal. Chem., 2009, 395, 1125 CrossRef CAS PubMed.
- M. Zhang, Z. Zhang, Y. Liu, X. Yang, L. Luo, J. Chen and S. Yao, Chem. Eng. J., 2011, 178, 443 CrossRef CAS PubMed.
- Y. Ren, M. Zhang and D. Zhao, Desalination, 2008, 228, 135 CrossRef CAS PubMed.
- X. Luo, S. Luo, Y. Zhan, H. Shu, Y. Huang and X. Tu, J. Hazard. Mater., 2011, 192, 949 CrossRef CAS PubMed.
- B. Guo, F. Deng, Y. Zhao, X. Luo, S. Luo and C. Au, Appl. Surf. Sci., 2014, 292, 438 CrossRef CAS PubMed.
- F. Aboufazeli, H. R. L. Z. Zhad, O. Sadeghi, M. Karimi and E. Najafi, Food Chem., 2013, 141, 3459 CrossRef CAS PubMed.
- T. P. Rao, R. Kala and S. Daniel, Anal. Chim. Acta, 2006, 578, 105 CrossRef CAS PubMed.
- U. Latif, A. Mujahid, A. Afzal, R. Sikorski, P. A. Lieberzeit and F. L. Dickert, Anal. Bioanal. Chem., 2011, 400, 2507 CrossRef CAS PubMed.
- S. Dai, M. C. Burleigh, Y. H. Ju, H. J. Gao, J. S. Lin, S. J. Pennycook, C. E. Barnes and Z. L. Xue, J. Am. Chem. Soc., 2000, 122, 992 CrossRef CAS.
- F. Dickert, P. Achatz and K. Halikias, Fresenius' J. Anal. Chem., 2001, 371, 11 CrossRef CAS.
- F. Xie, H. Xuan, Y. Ge, Y. Wang, T. Cao and K. Zhang, Chin. J. Anal. Chem., 2011, 39, 77 CrossRef CAS.
- F. Xie, G. Liu, F. Wu, G. Guo and G. Li, Chem. Eng. J., 2012, 183, 372 CrossRef CAS PubMed.
- J. Otero-Romaní, A. Moreda-Piñeiro, P. Bermejo-Barrera and A. M. Esteban, Microchem. J., 2009, 93, 225 CrossRef PubMed.
- J. W. Kim, J. Y. Ko, J. B. Jun, I. S. Chang, H. H. Kang and K. D. Suh, Colloid Polym. Sci., 2003, 281, 157 CAS.
- H. B. Hu, Z. H. Wang and L. Pan, J. Alloys Compd., 2010, 492, 656 CrossRef CAS PubMed.
- E. Birlik, A. Ersoz, E. Acykkalp, A. Denizli and R. Say, J. Hazard. Mater., 2007, 140, 110 CrossRef CAS PubMed.
- H. T. Fan, J. Li, Z. C. Li and T. Sun, Appl. Surf. Sci., 2012, 258, 3815 CrossRef CAS PubMed.
- G. Z. Fang, J. Tan and X. P. Yan, Anal. Chem., 2005, 77, 1734 CrossRef CAS PubMed.
- A. Otero-Romaní, A. Moreda-Piñeiro, P. Bermejo-Barrera and A. Martin-Esteban, Talanta, 2009, 79, 723 CrossRef PubMed.
- M. G. Segatelli, V. S. Santos, A. B. T. Presotto, I. V. P. Yoshida and C. R. T. Tarley, React. Funct. Polym., 2010, 70, 325 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |