Enhanced fluorescence of europium-doped yttrium hydroxide nanosheets modified by 2-thenoyltrifluoroacetone
Lin Zhanga,
Danyu Jiangb,
Jinfeng Xiab,
Na Zhangc and
Qiang Li*a
aDepartment of Chemistry, East China Normal University, Shanghai 200062, P. R. China. E-mail: qli@chem.ecnu.edu.cn
bShanghai Institute of Ceramics, Chinese Academy of Sciences, Shanghai 200050, P. R. China
cDepartment of Materials Engineering, Shanghai Institute of Technology, Shanghai 200235, P. R. China
First published on 7th April 2014
Abstract
A novel luminescent inorganic/organic hybrid nanosheet [Y2(1−x)Eu2x(OH)5(TTA)n]+ was successfully synthesized. The photoluminescence of the hybrid nanosheet was significantly different from that of the unmodified nanosheet. The main charge transfer band Eu–O of the nanosheet was replaced by the more efficient π–π* electron transition band of the organic ligands in the hybrid. The emission bands of the 5D0–7F2 electric dipole transition in the hybrid were enhanced greatly. We also prepared a novel transparent thin film of the luminescent inorganic/organic hybrid on FTO glass by electrophoretic deposition. On being excited by ultraviolet radiation, the thin films show strong red emission.
Exfoliation of layered materials into single layers or nanosheets of only a few layers has been exploited as a route to make nanosheets. Nanosheets display unique chemical and physical properties, which result from unusual structural features such as excellent two-dimensional anisotropy, which in turn results from the extremely small thickness of these nanosheets, of around 1 nm. With the emergence of nanosheets, intensive research has been directed towards the development of nanodevices.1–7 Most nanosheets synthesized so far are “macro-anions” bearing a negative charge.8–10 The appearance of positively charged nanosheets supplied a new way for hybrids, a representative example being exfoliated layered double hydroxides (LDHs) in water or organic solvents, have been reported over the past several decades.11–14 Recently, a new family of layered rare-earth hydroxides (LRHs) has attracted considerable attention science lanthanide elements, which is the base of a rich pool of functionalities,15–19 and the structural of LRHs is similarity to the well-known LDH. They have found numerous applications in optical devices, catalysis, molecular recognition, and medical uses.20–23 They can even be used as a building block for the fabrication of a wide variety of functional nanostructured materials.24–27
However, previous studies on LRHs have been mainly focused on their exfoliation and fluorescence property. However, one problem that remains associated with LRHs is that their fluorescence emissions are not strong enough; therefore, their utility in various applications is limited, because water molecules directly coordinated to the lanthanide metal centers can induce a drastic quenching effect on their emissions.28 One way to overcome this disadvantage is to remove water molecules by post-treating the LRHs by calcination. However, the post-treatment might destroy the special structure of the nanosheets owing to the high temperature used during calcination. Therefore, the unique single-layer structure of rare earth hydroxide nanosheets allowed us to develop a new method to replace the coordinated water molecules with organic ligands without damaging the nanosheet structure. Special inorganic/organic hybrid could also be obtained using this method.
In this paper, we report for the first time a method to enhance the photoluminescence properties of europium-doped yttrium hydroxide nanosheets modified by organic ligands. The as-obtained inorganic/organic hybrid show special photoluminescence spectra and can be used to produce well-defined transparent thin films on FTO (fluorine doped tin oxide) glass by electrophoretic deposition. On being excited with ultraviolet radiation, the thin films showed strong red emissions.
The layered material Y1.9Eu0.1(OH)5NO3·2H2O was synthesized via a hydrothermal reaction reported previously.16–19 The exfoliation was achieved by adding Y1.9Eu0.1(OH)5NO3·2H2O (0.5 g) to 100.0 mL of n-butanol. This yielded translucent colloidal solutions after repeated ultrasonic treatments of the mixtures for 30 minutes, suggesting the occurrence of delamination. After the unexfoliated residue was removed by centrifugation at 5000 rpm for 15 min, the resulting colloidal suspension was stable. No sediment was observed even after the suspension was left to stand for even one month.
The modified nanosheets were prepared as follows: during the exfoliation process, 0.01 g of 2-thenoyltrifluoroacetone (TTA) was dissolved in 100.0 mL of n-butanol, followed by the addition of 0.5 g of Y1.9Eu0.1(OH)5NO3·2H2O to the solution. A solution comprising inorganic/organic hybrid nanosheets was obtained after 30 min of ultrasonic treatments and 15 min of centrifugation at 5000 rpm. The thin film of nanosheets or nanosheets/TTA hybrid was prepared on FTO glass by electrophoretic deposition under 60 V for 10 min, in accordance with the positive charge of the LRHs surface.
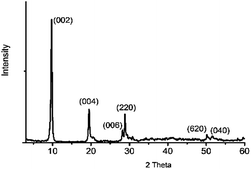
The phase purity of LRH was confirmed by powder X-ray diffraction. Fig. 1 shows the powder X-ray diffraction (XRD) pattern of the hydrothermal product Y1.9Eu0.1(OH)5NO3·2H2O. A series of strong (00l) reflections, previously reported to be characteristic of a layered phase,29 were observed.
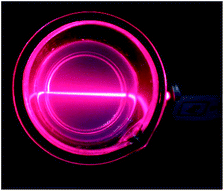
A colloidal solution of nanosheets was produced from as-synthesized LRHs by exfoliation. Fig. 2 shows the clear Tyndall light scattering of the colloidal suspension, suggesting the occurrence of delamination as a result of the supersonic treatment.
The ultra-thin and homogeneous nature of the nanosheets and nanosheets/TTA was confirmed by the transmission electron microscopy (TEM) pattern shown in Fig. 3a and c. Some sheet-like objects of irregular shapes can be observed in Fig. 3. The lateral dimension of the sheets was about several hundred nanometers. The images in Fig. 3b and d were selected area electron diffraction (SAED) patterns of the nanosheets and nanosheets/TTA, respectively. Both the SAED patterns displayed well-arranged spots, suggesting that the structure of the yttrium hydroxide layers is retained after exfoliation. The inhibited growth direction of the nanosheets can be determined to be (001) because the nanosheets with a large area lie along the Cu grid. The results provided strong evidence that the intralayer crystalline structure of Y1.9Eu0.1(OH)5NO3·2H2O was unchanged after exfoliation. The same SAED pattern in Fig. 3b and d indicates that the 2-thenoyltrifluoroacetone modification did not change the structure of the nanosheets.
 | ||
| Fig. 3 Transmission electron microscopy and selected area electron diffraction patterns of nanosheets (a and b) and nanosheets/TTA (c and d). | ||
The atomic force microscopy (AFM) image in Fig. 4 shows two-dimensional ultrathin sheets with lateral dimensions up to several hundred nanometers, although some smaller fragments were also observed. The thickness of nanosheet displayed in height profile was near about 1.1 nm, corresponding to a single layer of the layered Eu hydroxide, demonstrating a mono-layer of the nanosheet.
 | ||
| Fig. 4 Typical AFM image of the Y/Eu hydroxide nanosheets deposited on a Si substrate. The height profile along the broken line is shown in the right panel. | ||
The photoluminescence properties of LRHs solid have been widely reported.30,31 However, our attention was attracted to an interesting phenomenon, whereby the fluorescence intensity of the nanosheets could be greatly enhanced when the organic ligand HTTA was added. Fig. 5 displays the room-temperature excitation and emission spectra of the nanosheets and nanosheets/TTA suspensions. In the excitation spectra of the nanosheets suspension, the broad band at 285 nm is generated from the charge transfer between O2− and Eu3+, and the sharp band about 396 nm is ascribed to 7F0–5L6 of Eu3+.32–34 In the excitation spectra of the nanosheets/TTA suspension, the high intensity band at 377 nm is assigned to the π–π* electron transition of TTA, which was considered to be due to a more efficient energy transfer from the ligand to the central metal atom. The excitation band at 377 nm also confirms that TTA is coordinated to the rare earth ions of the nanosheets. The emission spectra consist of typical 5D0–7FJ (J = 1–4) transitions. From the emission spectra of the nanosheets/TTA suspension, it can be seen that the luminescent intensity of the 5D0–7F2 peak (electric dipole transition) is greatly increased.
 | ||
| Fig. 5 Room-temperature excitation and emission spectra of (a) nanosheets/TTA suspension and (b) nanosheets suspension. | ||
The increase in fluorescent intensity could be ascribed to two reasons. One reason was that H2O coordinated to Eu3+ in the nanosheets was substituted by the organic ligand TTA, reducing the non-radiative transition generated from thermal vibration and enhancing the emission. The other reason was an effective intra-molecular energy transfer from the ligands to the metal ions under near-UV light excitation. The relative intensities of the different transitions depend on the symmetry of the Eu3+ environment. The 5D0–7F2 transition is a typical electric dipole transition and varies greatly with the local symmetry of the Eu3+ ions, while the 5D0–7F1 transition corresponds to a parity allowed magnetic dipole transition and is in fact independent of the host material. Therefore, the enhanced red emission intensity resulting from the electric dipole transition of 5D0–7F2 at 616 nm indicates that the Eu3+ ions are located in an environment without inversion symmetry after the rare earth ions are coordinated by TTA. This result improves the monochromaticity of the red emission in the nanosheets/TTA hybrid (Fig. 6).
 | ||
| Fig. 6 The strong red light emission of (a) nanosheets film, (b) nanosheets/TTA film; transmission picture of (a) nanosheets film without TTA; (b) nanosheets/TTA film (c) FTO substrate. | ||
This kind of special inorganic/organic hybrid shows special photoluminescence properties and might be widely applied in lighting, optoelectronics, biological assays, and bio-imaging. An attractive application of the thin film of rare-earth compounds is in display devices. It is worth considering the preparation of luminescent thin films of positively charged nanosheets via electrophoretic deposition. Uniform thin films were fabricated on the FTO substrate from the nanosheets/TTA suspension. Comparing transmission picture (a–c), it is obvious that the nanosheets/TTA film displays fine fluorescence properties and great light transmission properties.
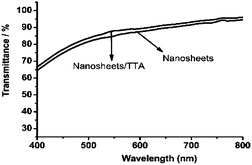
Next, we recorded the UV-Vis absorption spectra of the films. The curves in Fig. 7 indicate the good transmittance of the nanosheet/TTA thin films in the visible region of the electromagnetic spectrum.
The room-temperature excitation and emission spectra of the films are displayed in Fig. 8. No apparent difference exists between the spectra in Fig. 5a and 8a, indicating that the structure of the nanosheets was preserved in the film. All these results suggested a bright prospect for the applicability of these nanosheets.
 | ||
| Fig. 8 Room-temperature excitation and emission spectra of (a) nanosheets/TTA thin film on FTO glass and (b) nanosheets thin film. | ||
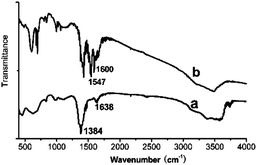
Finally, powders of the nanosheets and nanosheets/TTA hybrid, collected by scraping the as-obtained thin film on FTO glass, were used in FT-IR experiments to determine the structure of the complex. Fig. 9a displays the FT-IR spectra of the nanosheets, and Fig. 9b shows the spectra of the nanosheets/TTA hybrid. The sharp and strong absorption peak at 1384 cm−1 (Fig. 9a) is characteristic of an uncoordinated nitrate anion. The broad absorption peaks at about 3356 cm−1 and the shallow shoulder near 1638 cm−1 provide evidence for the presence of water molecules in the structure, and they are assigned to the O–H stretching vibrations (ν1 and ν3) and the H–O–H bending mode (ν2) respectively. The absorption band observed in the range 3400–3750 cm−1 (centered at 3603 cm−1) is indicative of hydroxyl (OH−) groups. In the spectra shown in Fig. 9b, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations shift from 1659 (close to thienyl) and 1632 cm−1 (close to trifluoromethyl) in the free HTTA to 1600 and 1547 cm−1, respectively, in the nanosheets/TTA complex, suggesting that the metal ions of Eu3+ were coordinated with TTA.35 It is well known that TAA ligands often form bidentate chelates with rare earth ions, while in the layered rare earth hydroxides, water molecules are mono- coordinated to the rare earth ions, depending on the surrounding of the rare earth metal in the nanosheet.16 After the top layer of H2O molecules were replaced, there was enough space for TTA to form a bidentate chelate by coordinating to the rare earth metal; this could be confirmed by infrared spectroscopy. The structure of the nanosheets were unchanged. The chelate did not bring about a change in the structure; this could be confirmed by the SEDA pattern in Fig. 3b and d, in which both the diffraction spots have the same distance to the center, suggesting that the structure of the yttrium hydroxide layers is retained in the nanosheets/TTA hybrid.
O stretching vibrations shift from 1659 (close to thienyl) and 1632 cm−1 (close to trifluoromethyl) in the free HTTA to 1600 and 1547 cm−1, respectively, in the nanosheets/TTA complex, suggesting that the metal ions of Eu3+ were coordinated with TTA.35 It is well known that TAA ligands often form bidentate chelates with rare earth ions, while in the layered rare earth hydroxides, water molecules are mono- coordinated to the rare earth ions, depending on the surrounding of the rare earth metal in the nanosheet.16 After the top layer of H2O molecules were replaced, there was enough space for TTA to form a bidentate chelate by coordinating to the rare earth metal; this could be confirmed by infrared spectroscopy. The structure of the nanosheets were unchanged. The chelate did not bring about a change in the structure; this could be confirmed by the SEDA pattern in Fig. 3b and d, in which both the diffraction spots have the same distance to the center, suggesting that the structure of the yttrium hydroxide layers is retained in the nanosheets/TTA hybrid.
Conclusions
In summary, we directly exfoliated the layered Y2(1−x)Eu2x(OH)5NO3·2H2O compound via ultrasonic treatments in n-butanol to prepare europium-doped yttrium hydroxide nanosheets. Due to their special single-layer structure, modification of the nanosheets with organic ligands of TTA could bring about a much more significant improvement in fluorescent intensity and monochromaticity. Furthermore, using the special inorganic/organic hybrid, we were able to synthesize a novel transparent thin film, which displayed strong red emission, by electrophoretic deposition.Notes and references
- C. Nethravathi, S. Sen, N. Ravishankar, M. Rajamathi, C. Pietzonka and B. Harbrecht, J. Phys. Chem. B, 2005, 109, 11468 CrossRef CAS PubMed.
- T. Sasaki, M. Watanabe, H. Hashizume, H. Yamada and H. Nakazawa, J. Am. Chem. Soc., 1996, 118, 8329 CrossRef CAS.
- Q. Zhu, J.-G. Li, C. Zhi, X. Li, X. Sun, Y. Sakka, D. Golberg and Y. Bando, Chem. Mater., 2010, 22, 4204 CrossRef CAS.
- R. Ma, Z. Liu, L. Li, N. Iyi and T. Sasaki, J. Mater. Chem., 2006, 16, 3809 RSC.
- J. T. Rajamathi, A. Arulraj, N. Ravishankar, J. Arulraj and M. Rajamathi, Langmuir, 2008, 24, 11164 CrossRef CAS PubMed.
- C. Nethravathi and M. Rajamathi, Carbon, 2008, 46, 1994 CrossRef CAS PubMed.
- L. Hu, R. Ma, T. C. Ozawa and T. Sasaki, Chem.–Asian J., 2010, 5, 248 CrossRef CAS PubMed.
- M. Alexandre and P. Dubois, Mater. Sci. Eng., R, 2000, 28, 1 CrossRef.
- J. Heising and M. G. Kanatzidis, J. Am. Chem. Soc., 1999, 121, 638 CrossRef CAS.
- N. Sukpirom and M. M. Lerner, Chem. Mater., 2001, 13, 2179 CrossRef CAS.
- A.-P. Mariko, F. Claude and J.-P. Besse, Chem. Commun., 2000, 91 Search PubMed.
- T. Hibino, Chem. Mater., 2004, 16, 5482 CrossRef CAS.
- Q. Wu, A. Olafsen, Ø. B. Vistad, J. Rootsc and P. Norby, J. Mater. Chem., 2005, 15, 4695 RSC.
- S. Britto, J. T. Rajamathi, N. Ravishankar, C. Shivakumara and V. Rajamathi, Solid State Sci., 2006, 8, 162 CrossRef CAS PubMed.
- L. J. McIntyre, L. K. Jackson and A. M. Fogg, Chem. Mater., 2008, 20, 335 CrossRef CAS.
- F. Geng, H. Xin, Y. Matsushita, R. Ma, M. Tanaka, F. Izumi, N. Iyi and T. Sasaki, Chem.–Eur. J., 2008, 14, 9255 CrossRef CAS PubMed.
- F. Geng, Y. Matsushita, R. Ma, H. Xin, M. Tanaka, F. Izumi, N. Iyi and T. Sasaki, J. Am. Chem. Soc., 2008, 130, 16344 CrossRef CAS PubMed.
- L. Poudret, T. J. Prior, L. J. McIntyre and A. M. Fogg, Chem. Mater., 2008, 20, 7447 CrossRef CAS.
- K.-H. Lee and S.-H. Byeon, Eur. J. Inorg. Chem., 2009, 929 CrossRef CAS.
- L. Hu, R. Ma, T. C. Ozawa, F. Geng, N. Iyi and T. Sasaki, Chem. Commun., 2008, 4897 RSC.
- L. Hu, R. Ma, T. Ozawa and T. Sasaki, Angew. Chem., Int. Ed., 2009, 48, 3846 CrossRef CAS PubMed.
- F. Gandara, E. G. Puebla, M. Iglesias, D. M. Proserpio, N. Snejko and M. A. Monge, Chem. Mater., 2009, 21, 655 CrossRef CAS.
- Y.-S. Yoon, B.-I. Lee, K. S. Lee, G. H. Im, S.-H. Byeon, J. H. Lee and I. S. Lee, Adv. Funct. Mater., 2009, 19, 3375 CrossRef CAS.
- V. Hornok, A. Erdőhelyi and I. Dékány, Colloid Polym. Sci., 2006, 284, 611 CAS.
- S. Huang, H. Peng, W. W. Tjiu, Z. Yang, H. Zhu, T. Tang and T. Liu, J. Phys. Chem. B, 2010, 114, 16766 CrossRef CAS PubMed.
- S. W. Keller, H.-N. Kim and T. E. Mallouk, J. Am. Chem. Soc., 1994, 116, 8817 CrossRef CAS.
- R. E. Schaak and T. E. Mallouk, Chem. Mater., 2000, 12, 3427 CrossRef CAS.
- Q. Zhu, J. G. Li, C. Zhi, X. Li, X. Sun, Y. Sakka, D. Golberg and Y. Bando, Chem. Mater., 2010, 22, 4204 CrossRef CAS.
- L. J. McIntyre, L. K. Jackson and A. M. Fogg, J. Phys. Chem. Solids, 2008, 69, 1070 CrossRef CAS PubMed.
- F. Geng, Y. Matsushita, R. Ma, H. Xin, M. Tanaka, N. Iyi and T. Sasaki, Inorg. Chem., 2009, 48, 6724 CrossRef CAS PubMed.
- X. Wu, J.-G. Li, Q. Zhu, J. Li, R. Ma, T. Sasaki, X. Li, X. Sun and Y. Sakka, Dalton Trans., 2012, 41, 1854 RSC.
- M. Yu, J. Lin and S. B. Wang, Appl. Phys. A, 2005, 80, 353 CrossRef CAS PubMed.
- M. Nichkova, D. Dosev, S. J. Gee, B. D. Hammock and I. M. Kennedy, Anal. Chem., 2005, 77, 6864 CrossRef CAS PubMed.
- Y. C. Kang, S. B. Park, I. W. Lenggoro and K. Okuyama, J. Phys. Chem. Solids, 1999, 60, 379 CrossRef CAS.
- M. Fernandes, V. Z. Bermudez, R. A. S. Ferreira, L. D. Carlos, A. Charas, J. Morgado, M. M. Silva and M. J. Smith, Chem. Mater., 2007, 19, 3892 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |