A one-step strategy for cross-linkable aliphatic polycarbonates with high degradability derived from CO2, propylene oxide and itaconic anhydride†
Pengfei Song*,
Xudong Mao,
Xuefeng Zhang,
Xiaogang Zhu and
Rongmin Wang
Key Laboratory of Eco-Environment-Related Polymer Materials of Ministry of Education, Key Laboratory of Polymer Materials of Gansu Province, College of Chemistry and Chemical Engineering, Northwest Normal University, Lanzhou 730070, P. R. China. E-mail: songpf@nwnu.edu.cn; Fax: +86-931-7972081; Tel: +86-931-7970358
First published on 18th March 2014
Abstract
A one-step strategy for the preparation of cross-linkable aliphatic polycarbonates via the direct terpolymerization of CO2, propylene oxide and itaconic anhydride is presented for the first time in this paper. The resulting terpolymers exhibit high degradability, and superior thermal and mechanical performance compared with poly(carbonate propylene).
Carbon dioxide (CO2) is one of the main greenhouse gases, and also an inexpensive C1-feedstock. Thus, the utilization of abundant, non-toxic and non-flammable CO2 has attracted increasing attention recently as an effective approach to reduce the release of the greenhouse gases.1 The alternating copolymerization of CO2 and epoxides, which was first reported by Inoue and co-workers in 1960s,2 has been considered as one of the most promising processes to produce a variety of aliphatic polycarbonates.3 Among these, poly(propylene carbonate) (PPC) derived from CO2 and propylene oxide (PO) is known as a cheap and biodegradable polymer material due to its good properties such as compatibility, translucence, innocuousness etc.4 PPC has great potential application in the development of plastic, elastomer, fiber, adhesive, and so on. However, the practical application of PPC has been limited by its poor thermal stability and mechanical performance.5
Therefore, many approaches have been developed to improve the thermal and mechanical properties of PPC, such as physical blending,6 terpolymerization7 and cross-linking.8,9 Although several attempts have been achieved, it is now very hard to modify the material profile of PPC fitting for existing applications. In fact, physical blending often meets with an incompatibility between the two phases of PPC and other polymers, organic compounds or inorganic fillers.10 In addition, preparation of terpolycarbonate major depends on the activities of co-monomer and catalyst, leading to the increase of cost.11 Based on this, the cross-linking technique has become a quite effective method to improve the thermal and mechanical performance of PPC. In our earlier work, cross-linkable aliphatic polycarbonates prepared from the copolymerization of CO2, PO and maleic anhydride using dicumyl peroxide (DCP) as a cross-linking agent exhibited superior thermal stability and mechanical performance. However, it was difficult to remain the degradability of PPC after cross-linking.8 Moreover, the strategies published for cross-linking require two steps including the incorporation of double bonds into PPC chains and the post-treatment of PPC with radical initiators, which is uneconomical.9
Recently, a one-step strategy has developed by the terpolymerization of CO2, PO and pyromellitic dianhydride (PMDA) to improve the thermal and mechanical properties of PPC, although the resulting terpolymers exhibited superior thermal and mechanical performance compared with PPC, it is environmentally unfriendly due to the toxicity of PMDA.12 In contrast, itaconic anhydride (IAn) is one of nontoxic, environmentally friendly and renewable resource, can be used to prepare polymer materials by ring-opening polymerization or radical polymerization.13,14 Although a variety of acid anhydride has been introduced into the copolymerization of CO2 and epoxides to improve the thermal properties and degradability of aliphatic polycarbonates,15 to the best of our knowledge, there has been no report on the terpolymerizaton of CO2, epoxides and IAn until now, especially for the preparation of cross-linkable aliphatic polycarbonate. Thus, we report here a one-step facile strategy for cross-linkable PPC by the direct terpolymerization of CO2, PO and IAn was carried out using zinc glutarate (ZnGA) to enhance the mechanical and thermal performance of PPC. The gel content and properties of terpolymer (PPCIAn) derived from CO2, PO and IAn in different polymerization conditions were discussed. Interestingly, PPCIAns exhibit superior degradability compared with PPC.
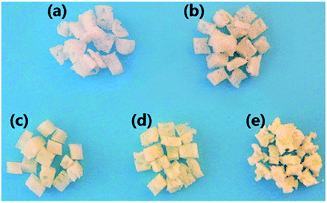
The synthetic strategy developed to obtain cross-linkable aliphatic polycarbonate is illustrated in Scheme 1. PPCIAns were synthesized in one-step by introducing IAn into the copolymerization between CO2 and PO. The addition of IAn into terpolymerization was varied between 0 and 5 mol% relative to PO, in removal of the catalyst in polymers after polymerization, the precipitates of either neat PPC or PPCIAns solution of chloroform from ethanol are whole mass polymers, which was dried and cut with scissors and seems like (a)–(e) in Fig. 1. (a) showed PPC special white, but PPCIAns including (b)–(e) appear yellow. Moreover, the colour of PPCIAns become darker with the increasing content of IAn, corresponding to the decrease of the solubility for PPCIAns in many solvents including chloroform, tetrahydrofuran, acetone and dimethyl sulfoxide, indicating that the cross-linking structure of PPCIAns derived from the copolymerization of CO2, PO and IAn. It is ascribe to the exo-type double bond of IAn unit in the backbone of PPCIAns can be cross-linked by thermal initiation under copolymerization conditions.
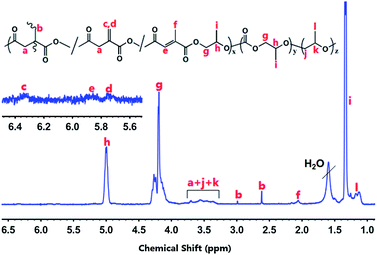
The FT-IR spectra (Fig. S1†) directly proves that IAn is chemically incorporated into polymers successfully and the cross-linking structure of PPCIAn further confirmed by 1H NMR spectroscopy (Fig. 2 and S2†). Compared with PPC, the new characteristic absorptions at 1642.5 cm−1 is assigned as the unsaturated double bonds stretching vibration. From 1H NMR spectroscopy, the absorption peaks at 1.3, 4.2 and 5.0 ppm were assigned to CH3, CH2 and CH in the carbonate unit, respectively; two weak peaks at 5.77 and 6.33 ppm appeared which was assigned to the two protons of exo-type double bonds, indicating that the IAn units were incorporated into the PPC backbone successfully. Furthermore, cross-linking was supported by appearance of peaks at 2.62 and 2.99 ppm assigned to methylene protons generated by the cross-linking of exo-type double bonds in IAn unit. Meanwhile, peaks ascribed to methyl protons (2.06 ppm) and methine protons (5.85 ppm) of citraconic anhydride (CAn) units were observed unexpectedly, demonstrating that the isomerization from IAn unit into CAn unit occurred under the experimental conditions. Peaks at 3.30–3.75 ppm, in addition to methylene protons in IAn unit, there are peaks characteristics of ether linkages were very weak, indicating the alternating structure of terpolymers derived from CO2, PO and IAn.
PPCIAns were synthesized by the copolymerization of CO2, PO, and IAn under ZnGA catalyst and the results are listed in Table 1. The yield of the copolymerization was between 6205.7 and 8036.3 g mol−1 Zn, corresponding to IAn/PO molar feed ratios varying from 0 to 0.05. It was encouraging that IAn can improve the yields when a small amount of IAn was added to the polymerization system. As can be seen in Table 1, Mn of PPCIAns are higher than that of common PPC. In particular, Mn of PPCIAn4 was extremely high, up to 101 kg mol−1. So the addition of IAn has a very obvious advantage of increasing yields and average molecular weights. These beneficial phenomena show a superior difference from previous reports and certify that the soluble polymers are most the uncross-linked PPC with a very low ratio of IAn incorporated, for only this structure model makes the molecule have both the chloroform solubility and a large Mn with improvement above 200% compared with that of PPC. The gel contents of PPCIAns increased with the increase amount of IAn introduced into the copolymerization of CO2 and PO. Furthermore, the gel contents of PPCIAns showed 0–60.1%, which could be adjusted easily by controlling polymerization conditions such as temperature, time and CO2 pressure (Table S1†).
The glass transition temperature (Tg) of PPCIAns and PPC were measured by DSC. As shown in Fig. 3 and Table S2,† Tg of PPCIAns increase with increasing amount of IAn introduced in terpolymerization are all higher than that of PPC. Interestingly, PPCIAn3 and PPCIAn4 exhibit an endothermic broad peak at the high temperature of 97.6 °C and 90.4 °C as well as a low Tg of 39.4 °C and 39.7 °C, respectively. In fact, the insoluble part and the soluble part of PPCIAn have different backbone constituents as well as morphologies, leading to the network structure of the products. The other two products, PPCIAn1 and PPCIAn2, each exhibit only one Tg. This observation demonstrates that the excellent miscibility between the cross-linked CO2/PO/IAn terpolymers and the uncross-linked part is reached.
PPCIAns also exhibit superior thermal stability. As shown in Fig. 4 and Table S2,† the thermal decomposition temperature (5% weight loss temperature, Td,−5%; and maximum weight loss temperature, Td,max) for PPCIAns are much higher than those of PPC. PPCIAn3 exhibits very high thermal decomposition temperature for Td,−5% of 257.8 °C and Td,max of 286.1 °C, respectively. The thermal gravimetric (TG) curve of PPCIAn4 shows the further cross-linking on the double bond of IAn unit in the backbone, which favors to the process of PPCIAn.
The static mechanical properties of the resulted PPCIAn terpolymers and PPC were measured in terms of tensile strength and elongation at break. As shown in Fig. 5, the tensile strength of PPCIAns are all much higher than that of PPC. The highest tensile strength of PPCIAn4 reached 27.5 MPa, together with an elongation at break of 554.6%. The results also revealed that the tensile strength of PPCIAn increased with increasing gel content. This means that highly cross-linked PPCIAn terpolymers become strong enough as commercial ABS terpolymers.
Generally, we consider that cross-linking is an efficient method to increase thermal and mechanical performance of polymer but decrease its degradability. Thus, there has no publication on the degradability of cross-linked PPC until now. Encouraged by the reports of Nishimura,13 the degradability of PPCIAn4 with the gel content of 56.8% was determined in the phosphate buffer solution (PBS) of pH 7.4 at 37 °C for 10 weeks. Surprisely, as shown in Fig. S3,† PPCIAn4 exhibit high degradability compared with PPC. After 10 weeks, the weight loss increased up to 28.28%, which was significantly higher than that of PPC of 4.58%. Fig. 6 shows SEM images of PPC and PPCIAn4 taken before and after into PBS after 10 weeks. Apparently, compared with the morphology of original PPCIAn4 film Fig. 6b, numerous large cavities can be observed in Fig. 6d, in good accordance with the weight loss results as discussed above, which also evince that the degradation of PPCIAn4 begins from the surface. The high degradability of PPCIAn presumably arises from the polyester unit from the introducing of IAn into the backbone of PPC. The water sorption of aliphatic polycarbonates plays a key role to enhance the degradation rate in burial or immersion, generally used to evaluate the hydrophilicity of examined samples. As shown in Fig. S4,† it is obvious that the water sorption of PPCIAn4 much higher than those of PPC, which also indicate that the decrease in water sorption of PPCIAn4 after 5 weeks due to the degradation in PBS.
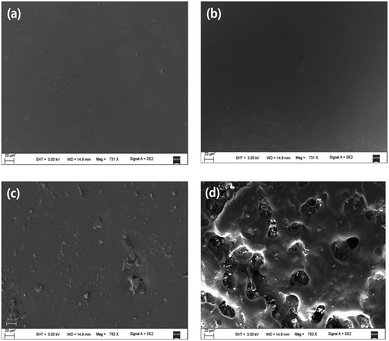 | ||
| Fig. 6 SEM images of PPC and PPCIAn4 (Table 1, entry 5) films after degradation: (a) PPC 0 weeks; (b) PPCIAn4 0 weeks; (c) PPC 10 weeks; (d) PPCIAn4 10 weeks. | ||
Conclusions
In conclusion, cross-linkable aliphatic polycarbonate PPCIAn was easily synthesized from CO2, PO and IAn by one-step strategy. PPCIAns with a small IAn addition of no more than 5 mol% relative to PO exhibit superior thermal, mechanical and degradation properties compared with PPC. Furthermore, the addition of IAn improves the product yields and molecular weights obviously. These findings reveal an effective and simple means to resolve the key issues for PPC to be used in the widely practical applications. Although water degradability was tested on terpolymers in this paper, we infer that PPCIAns could be showed better biodegradability in enzymatic degradation test. With its further study, this new material with excellent thermal, mechanical, and degradable properties is highly expected to be a real alternative to conventional plastics or biomaterials in wide application fields in the near future.Acknowledgements
This work was supported by Gansu Province Natural Science Foundation (no. 1208RJYA032), Project of Gansu Province Department of Education (no. 2013A-009) and Key Project of NWNU (no. NWNU-LKQN-12-4).Notes and references
- (a) M. Cokoja, C. Bruckmeier, B. Rieger, W. A. Herrmann and F. E. Kühn, Angew. Chem., Int. Ed., 2011, 50, 8510 CrossRef CAS PubMed; (b) T. Sakakura, J. C. Choi and H. Yasuda, Chem. Rev., 2007, 107, 2365 CrossRef CAS PubMed.
- S. Inoue, H. Koinuma and T. Tsuruta, J. Polym. Sci., Part B: Polym. Lett., 1969, 7, 287 CrossRef CAS.
- (a) D. J. Darensbourg and S. J. Wilson, Green Chem., 2012, 14, 2665 RSC; (b) X. B. Lu and D. J. Darensbourg, Chem. Soc. Rev., 2012, 41, 1462 RSC; (c) S. Klaus, M. W. Lehenmeier, C. E. Anderson and B. Rieger, Coord. Chem. Rev., 2011, 255, 1460 CrossRef CAS; (d) D. J. Darensbourg, Chem. Rev., 2007, 107, 2388 CrossRef CAS PubMed; (e) J. Hilf, P. Schulze, J. Seiwert and H. Frey, Macromol. Rapid Commun., 2014, 35, 198 CrossRef CAS PubMed; (f) J. Hilf, A. Phillips and H. Frey, Polym. Chem., 2014, 5, 814 RSC; (g) W. M. Ren, M. W. Liang, Y. C. Xu and X. B. Lu, Polym. Chem., 2013, 4, 4425 RSC; (h) X. J. Wu, H. Zhao, B. Nornberg, P. Theato and G. A. Luinstra, Macromolecules, 2014, 47, 492 CrossRef CAS; (i) R. J. Wei, X. H. Zhang, Y. Y. Zhang, B. Y. Du, Z. Q. Fan and G. R. Qi, RSC Adv., 2014, 4, 3188 RSC; (j) G. P. Wu, P. X. Xu, X. B. Lu, Y. P. Zu, S. H. Wei, W. M. Ren and D. J. Darensbourg, Macromolecules, 2013, 46, 2128 CrossRef CAS.
- G. A. Luinstra and E. Borchardt, Adv. Polym. Sci., 2012, 245, 29 CrossRef CAS.
- G. A. Luinstra, Polym. Rev., 2008, 48, 192 CrossRef CAS.
- (a) Y. S. Qin, L. J. Chen, X. H. Wang, X. J. Zhao and F. S. Wang, Carbohydr. Polym., 2011, 84, 329 CrossRef CAS; (b) S. B. Khan, J. Seo, E. S. Jang, K. Akhtar, K. I. Kim and H. Han, Macromol. Res., 2011, 19, 876 CrossRef CAS.
- (a) P. F. Song, M. Xiao, F. G. Du, S. J. Wang, L. Q. Gan, G. Q. Liu and Y. Z. Meng, J. Appl. Polym. Sci., 2008, 109, 4121 CrossRef CAS; (b) Y. L. Liu, M. Xiao, S. J. Wang, L. Xia, D. M. Hang, G. F. Cui and Y. Z. Meng, RSC Adv., 2014, 4, 9503 RSC.
- P. F. Song, S. J. Wang, M. Xiao, F. G. Du, Li. Q. Gan, G. Q. Liu and Y. Z. Meng, J. Polym. Res., 2009, 16, 91 CrossRef CAS.
- Y. H. Tao, X. H. Wang, X. J. Zhao, J. Li and F. S. Wang, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 5329 CrossRef CAS.
- Y. J. Li and H. Shimizu, ACS Appl. Mater. Interfaces, 2009, 1, 1650 CAS.
- M. R. Kember, A. Buchard and C. K. Williams, Chem. Commun., 2011, 47, 141 RSC.
- L. J. Gao and J. Y. Feng, J. Mater. Chem. A, 2013, 1, 3556 CAS.
- A. Takasu, M. Ito, Y. Inai, T. Hirabayashi and Y. Nishimura, Polym. J., 1999, 31, 961 CrossRef CAS.
- S. R. Shang, S. J. Huang and R. A. Weiss, Polymer, 2009, 50, 3119 CrossRef CAS.
- (a) S. Peng, Y. An, C. Chen, B. Fei, Y. Zhuang and L. Dong, Polym. Degrad. Stab., 2003, 80, 141 CrossRef CAS; (b) X. Ma, P. R. Chang, J. Yu and N. Wang, Carbohydr. Polym., 2008, 71, 229 CrossRef CAS; (c) Y. Liu, K. Huang, D. Peng and H. Wu, Polymer, 2006, 47, 8453 CrossRef CAS; (d) S. Huijser, E. H. Nejad, R. Sablong, C. Jong, C. E. Koning and R. Duchateau, Macromolecules, 2011, 44, 1132 CrossRef CAS; (e) D. J. Darensbourg, R. R. Poland and C. Escobedo, Macromolecules, 2012, 45, 2242 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and some characterization of PPCIAns are presented. See DOI: 10.1039/c4ra01514b |
| This journal is © The Royal Society of Chemistry 2014 |


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000
000

