Fabric-based flexible electrode with multi-walled carbon nanotubes@Ni network structure as a novel anode for hydrogen peroxide electrooxidation
Dongming Zhang,
Ke Ye,
Kui Cheng,
Yang Xu,
Jinling Yin,
Dianxue Cao and
Guiling Wang*
Key Laboratory of Superlight Materials and Surface Technology of Ministry of Education, College of Materials Science and Chemical Engineering, Harbin Engineering University, Harbin, 150001, P.R. China. E-mail: wangguiling@hrbeu.edu.cn; Fax: +86-451-82589036; Tel: +86-451-82589036
First published on 25th March 2014
Abstract
A simple method involving dyeing and electrodeposition is introduced to fabricate a three-dimensional Ni@multi-walled carbon nanotubes flexible electrode on wearable fabric. The as-prepared Ni@multi-walled carbon nanotubes/Fabric (Ni@MWNTs/Fabric) electrode was characterized by scanning electron microscopy and X-ray diffraction spectrometry. The catalytic activity of the Ni@MWNTs/Fabric electrode for hydrogen peroxide electrooxidation was tested by means of cyclic voltammetry and chronoamperometry. Such a three-dimensional hybrid electrode structure allows a large specific surface area and a large mass loading, which lead to a high areal current density of 720 mA cm−2 at 0.5 V in 2 mol dm−3 NaOH and 2.5 mol dm−3 hydrogen peroxide. The electrode shows great promise as the anode of a direct peroxide fuel cell due to its being flexible, wearable, and environmentally friendly.
1. Introduction
The direct peroxide–peroxide fuel cell (DPPFC) has attracted much attention due to its low cost, compactness, easy operation, being workable without air, providing both power and oxygen, and so on.1–7 As the anode fuel of a DPPFC, the electrooxidation of hydrogen peroxide (H2O2) has fast kinetics and involves no poisonous species,1–13 so it is a promising and environmentally friendly fuel. The study of the electrocatalysts for H2O2 electrooxidation has become a hot topic in recent years. Several types of catalyst for the electrooxidation of H2O2 have been developed. It has been reported previously that metals such as platinum,4,8–10 palladium,4,5 gold,4,5,11 silver12 and a combination of these5 exhibit effective catalytic performance, but high prices and limited resource restrict their extensive utilization. In order to reduce the amount of noble metals used and to reduce the cost of electrodes, transition metals, such as Ni and Co,4,6,7 have been employed to catalyze the H2O2 electrooxidation. However, they suffer the drawback of low catalytic activity. So, developing transition catalysts that can make H2O2 electrooxidation more effective is necessary and meaningful.An excellent substrate is one of the decisive factors that determine the catalytic activity of an electrode. And, then, the structure, conductivity, mechanical stability and price are the considerations that determine whether the collector is a promising material to apply in our daily life and industrial production. Metallic materials (e.g. aluminum foil, titanium plate, copper sheet)14–19 are the most widely used electrode material, due to their high electronic conductivity. Nowadays, carbon materials (e.g. carbon cloth, carbon paper)5,6,20–22 can be used as the collector, due to their deformability. Recently, three-dimensional (3D) structure electrodes (e.g. nickel foam, copper foam, titanium foam, sponge and some other foams and nano wires)7,23–30 have become a research hotspot, owing to their excellent mass transport properties, high catalyst utilization and capability of achieving large catalytic surface areas. Currently, energy storage devices based on conductive paper31 or textile32 with carbon nanotubes (CNTs) have attracted much attention. However, few of them have been employed as a flexible electrode substrate for DPPFC.
Fabrics, or the raw material of clothes, towels, bed sheets and cotton pads, are indispensable in our daily lives. In general, these fabrics are abandoned after being used for a period of time, which leads to a waste of resources and to environmental pollution. Most people ignore this phenomenon.
In this study, we fabricated a novel MWNTs/Fabric substrate by a simple “dipping and drying” method. The fabric was the cotton pads that are commonly used by women. MWNTs were wrapped around the fabric by van der Waals' force, and the MWNTs/Fabric exhibits a unique 3D network structure compared with the carbon cloth and carbon paper. Nano-scaled Ni particles were electrodeposited on the surface of the MWNTs/Fabric. The Ni@MWNTs/Fabric electrode avoids the use of metal resources in the electrode material, compared with the conventional metal electrode, and shows a remarkably high catalytic activity for H2O2 electrooxidation. The MWNTs/Fabric is also much cheaper than carbon cloth and such utilization of discarded fabrics reduces environmental pollution.
2. Experimental
Fabrication
The MWNTs/Fabric substrate is prepared by self-assembling of MWNT layers on a fabric. The fabric is the discarded cotton pads, which is commercially made up of polyester fibers and has a high hygroscopicity as a normal cloth (HAOLING Daily necessity & Cosmetics Co. Ltd.). In detail, a piece of fabric was first washed several times with acetone and ethanol and then dried at 373.15 K in a vacuum oven for 2 h prior to use. Then, the cleaned fabric was dipped into a ultrasonic uniform suspension containing 0.13 g MWNTs (>50 nm in outer diameter and 10–20 μm in length; Shenzhen Nanotech Port Co. Ltd.), 0.5 g sodium dodecyl benzene sulfonate (SDBS) and 50 mL ultrapure water (Milli-Q, 18 MΩ cm). After dipping for 30 s, the fabric was removed from the suspension and dried at 373.15 K for 2 h. The dip–dry cycle was repeated 5 times to obtain the MWNTs/Fabric substrate, which was further washed with a large amount of ultrapure water to remove SDBS. The Ni@MWNTs/Fabric electrode was prepared by electrodeposition of Ni on the surface of the MWNTs/Fabric, which was carried out in a standard three-electrode electrochemical cell using a computerized potentiostat (Autolab PGSTAT302, Eco Chemie) controlled by GPES software. A piece of MWNTs/Fabric substrate (10 × 10 mm) was employed as the working electrode, a platinum foil as the counter electrode, and a saturated Ag/AgCl, KCl electrode as the reference electrode. The electrodeposition was carried out on four pieces of MWNTs/Fabric at constant current densities of 5, 10, 15 and 20 mA cm−2, respectively, in a solution of 2 mol dm−3 NH4Cl and 0.1 mol dm−3 NiCl2. The electrodeposition time was kept constant at 4 h.Materials characterization
The morphology of the electrodes was determined using a scanning electron microscope (SEM, JEOL JSM-6480). The structure was analyzed by a powder X-ray diffractometer (XRD, Rigaku TTR-III) equipped with Cu Kα radiation (λ = 0.15406 nm).Electrochemical measurements
The electrochemical oxidation of H2O2 was measured by cyclic voltammetry and chronoamperometry in the same three-electrode electrochemical cell using NaOH as the electrolyte and the 1 cm2 Ni@MWNTs/Fabric electrode. All solutions were made with analytical grade chemical reagents and ultrapure water (Milli-Q 18 MΩ cm). All potentials were referred to the saturated Ag/AgCl, KCl reference electrode.3. Results and discussion
The fabrication process of the Ni@MWNTs/Fabric electrode via dyeing and electrodeposition is illustrated in Fig. 1. The original fabric is pure white, and, after dyeing of MWNTs and electrodeposition of Ni, the fabric becomes black and green, respectively. The MWNTs/Fabric substrate is about 0.0079 g and the mass reached 0.0155, 0.0228, 0.0272 and 0.0304 g after 4 h of electrodeposition at the constant current densities of 5, 10, 15 and 20 mA cm−2, respectively. The SEM images of the bare MWNTs/Fabric and the Ni@MWNTs/Fabric electrodes prepared at different electrodeposition current densities (5, 10, 15 and 20 mA cm−2) are shown in Fig. 2. At low magnification, it is obvious that the MWNTs/Fabric substrate, constituted by fabric lines (Fig. 2a), exhibits a 3D-network structure. The diameter of the single fabric liner is around 15 μm. At high magnification, we found that the single fabric line is composed of some detailed lines (Fig. 2b). The fabric line shows a plush stuffed surface, which indicates that the MWNTs were coated in the surface. The existence of MWNTs ensures good electrical conductivity (a sheet resistance of 1 Ω square−1, measured by a four points probe technique) of the MWNTs/Fabric substrate. Furthermore, the H2O2 can be absorbed by the fabric, which ensures full contact between the H2O2 and Ni during the reaction process. The surface of the fabric lines are encapsulated by the Ni after the electrodeposition process (Fig. 2c, e, g and i). In order to compare the Ni particles in the surface of the fabric line at different electrodeposition current densities (5, 10, 15 and 20 mA cm−2), the details of the four kinds of electrode surface are shown by Fig. 2d, f, h and j. As depicted in the figures, the diameters of the Ni particles prepared at 5 and 10 mA cm−2 were around 1100 nm and the diameter was over 1200 nm when the electrodeposition current density reached 20 mA cm−2. However, the diameters of the Ni particles prepared at 15 mA cm−2 were only around 700 nm. First, the diameter of the Ni nanoparticles is determined by the overpotential. When the overpotential increases, the diameter of the Ni nanoparticles will decrease. With an increase in the current density, the overpotential increases. So, the diameter of the Ni nanoparticles electrodeposited at 15 mA cm−2 was smaller than those at 5 and 10 mA cm−2. However, when the electrodeposition current density was higher (20 mA cm−2), the electric quantity was higher and the electrodeposition mount of the metallic Ni was more than under the other conditions (5, 10 and 15 mA cm−2), which is further demonstrated by the electrodeposition mass of the Ni particles (Fig. 1). Second, the metallic Ni is ferromagnetic and the massive Ni particles will aggregate at 20 mA cm−2, which leads to a bigger diameter of Ni particles. So, the Ni electrode prepared at 15 mA cm−2 exhibits the largest specific surface area. This will markedly increase the activity of the Ni owing to the higher surface energy compared to that of the other electrodes.The X-ray diffraction patterns of the Ni@MWNTs/Fabric electrodes are shown in Fig. 3. The scan range was set from 10° to 85°. It is obvious that the diffraction peaks between 15° and 30° are the substrate peaks. According to the JCPDS card no. 04-8450, the (111), (200) and (220) diffractions of cubic Ni are located at 2θ = 44°, 52° and 76°, respectively. It demonstrates that Ni presents in a metallic state of oxides or hydroxides. The intensity of the three Ni peak increased with an increase of the electrodeposition current density, which indicates that the content of the Ni in the Ni@MWNTs/Fabric increased.
The H2O2 electrooxidation activities on the Ni@MWNTs/Fabric electrodes with different electrodeposition current density are shown in Fig. 4. Fig. 4a–d present the electrodeposition current density range from 5 to 20 mA cm−2. All of the open circuit potentials (OCP) of the Ni@MWNTs/Fabric electrodes in H2O2 solution were around −0.16 V. The Ni@MWNTs/Fabric electrodes exhibited a strong oxidation peak at around 0.43 V and an obvious reduction peak at around 0.23 V in blank NaOH solution, which is attributed to the redox reactions of Ni2+/Ni3+ according to a previous report.4,6,7 The surface of the metallic Ni will be partly oxidized to Ni2+ in a solution with strong oxidization and the conversion of Ni2+/Ni3+ will occur at high potential. Furthermore, the existence of Ni2+ helps the negative valence oxygen in H2O2 to diffuse to the surface of the Ni electrode. Compared with the electrooxidation behavior in bare NaOH solution, the current density at the oxidation peak increased with an increase in the H2O2 solution, which demonstrates that the oxidation of H2O2 is controlled by diffusion at low H2O2 concentration. When the H2O2 concentration was higher than 1.0 mol dm−3, the oxidation peak of H2O2 disappeared. The highest current density at 0.5 V as a function of the electrodeposition current density is shown in Fig. 4e. The highest oxidation current density reached 340, 630, 720 and 640 mA cm−2 at 0.5 V in 2.0, 3.5, 2.5 and 3.0 mol dm−3 H2O2 solution, respectively, on the four electrodes. When the H2O2 concentration increased further, the current densities decreased.
A schematic diagram depicting the mechanism for the H2O2 electrooxidation on the Ni@MWNTs/Fabric electrodes is shown in Fig. 5. The catalytic performance of electrooxidation of H2O2 on the electrode is affected seriously by H2O2 concentration. When the H2O2 concentration is low, there are adequate Ni particles to act as the catalytic sites for H2O2 electrooxidation, and the O–O bond can be in full contact with Ni (style I in Fig. 5) and convert to O2 and release electrons [eqn (1)] (2–8). With increasing H2O2 concentration, all of the Ni catalytic sites will be occupied by O–O bonds and some of the surplus H2O2 can't be electrocatalyzed (style II in Fig. 5). When the H2O2 concentration is over high, most of the O–O bonds in H2O2 can't come into contact with Ni, which will cause a large amount of hydrolysis of H2O2 [eqn (2)] (style III in Fig. 5) (2–5), and the released O2 will be adsorbed on the surface of the Ni particles and inhibit contact between the Ni and H2O2. In addition, the released O2 may reduce the electronic conductivity of the reaction solution, which will also lead to poorer performance.
 | ||
| Fig. 5 Schematic diagram depicting the mechanism for the H2O2 electrooxidation on the Ni@MWNTs/Fabric electrode. | ||
When the concentration of the alkali liquor is fixed, the H2O2 at high concentration (0.25–2.0 mol dm−3) has almost no influence on the electrooxidation of H2O2 on Ni–carbon fiber6 and Ni–Ni foam7 electrodes. However, the catalytic performance of the H2O2 electrooxidation was markedly affected by the H2O2 concentration (0.20–2.5 mol dm−3) on the Ni@MWNTs/Fabric electrode, which is vital to change discharge current to satisfy industrial requirements. So, as a fuel for underwater and space power sources, H2O2 at various high concentrations can provide large amounts of oxygen for breathing, and also offer different discharge currents, both of which are more favorable than results from our previous studies.6,7
| H2O2 + 2OH− → 2H2O + O2 + 2e− | (1) |
| 2H2O2 → 2H2O + O2 | (2) |
Among the four electrodes, the highest catalytic performance was obtained with the electrode prepared at a constant electrodeposition current of 15 mA cm−2 (Fig. 4e), so we discuss the specific catalytic activity of H2O2 electrooxidation on this electrode below.
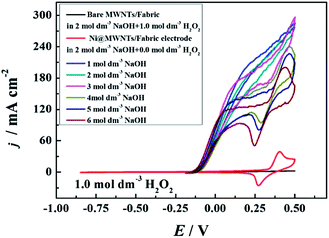
The effects of NaOH concentration are shown in Fig. 6. The concentration of H2O2 was fixed at 1.0 mol dm−3 and the concentration of NaOH varied from 1 to 6 mol dm−3. All of the onset potentials were around −0.16 V. At low NaOH concentrations (1 and 3 mol dm−3), the current density reached around 270 mA cm−2 and there was no obvious reduction peak in the CV curves. In contrast, the reduction peaks gradually shifted in the negative direction when the NaOH concentration increased from 3 to 6 mol dm−3. Clearly, the current densities decreased significantly when the concentration of NaOH was higher than 3 mol dm−3. The over-high NaOH concentration reduced the concentration of H2O2 that could diffuse to the surface of the electrode. It was also obvious that the MWNTs/Fabric had no catalytic activity for the H2O2 electrooxidation.
The stability of the Ni@MWNTs/Fabric electrode for H2O2 electrooxidation was investigated by chronoamperometric measurements in 2 mol dm−3 NaOH and 2.0 mol dm−3 H2O2. The results are shown in Fig. 7. The potentials were selected in the region according to the polarization curves in Fig. 4. At low potentials (−0.05 V and 0.05 V), the oxidation current density reached a steady-state quickly after about 50 s and kept stable during the 20 min test period. However, when the constant potential was applied at 0.15 V, the CA curve became rough and the current density decreased slightly during the reaction process. This phenomenon may be caused by the large amount of oxygen bubbles in the surface of the electrode and the consumption of the H2O2 at high oxidation potential.7
 | ||
| Fig. 7 Chronoamperometric curves for H2O2 electrooxidation at different potentials in 2 mol dm−3 NaOH and 2.0 mol dm−3 H2O2. | ||
The reaction temperature has great influence on the electrochemical behavior of H2O2 electrooxidation. It can be seen, from Fig. 8a, that the onset potentials moved a little more negatively and the oxidation current densities increased obviously with increasing the temperature. The activation energy for the electrooxidation of H2O2 on the Ni@MWNTs/Fabric electrode was calculated to be −11.64 KJ mol−1, obtained from the Arrhenius relationship (eqn (3)),7 where j is the current density, T is the thermodynamic temperature, R is the molar gas constant, and Ea is the activation energy. The logarithm of peak current densities (ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) j) at 0.46 V plotted against the reciprocal of absolute temperature (1/T) is shown in Fig. 8b.
j) at 0.46 V plotted against the reciprocal of absolute temperature (1/T) is shown in Fig. 8b.
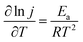 | (3) |
4. Conclusions
In summary, a novel Ni@MWNTs/Fabric electrode assembled by simple direct infiltration and electrodeposition methods was employed to catalyze the electrooxidation of H2O2. The Ni@MWNTs/Fabric electrode exhibited a 3D network structure and allowed a large mass loading of Ni particles. The oxidation current density reached 720 mA cm−2 in 2.0 mol dm−3 NaOH and 2.5 mol dm−3 H2O2 at 0.5 V. The activation energy for the electrooxidation of H2O2 on the Ni@MWNTs/Fabric electrode was calculated to be −11.64 KJ mol−1.Acknowledgements
We gratefully acknowledge the finance supported by the Fundamental Research Funds for the Central Universities (HEUCF20130910013 and HEUCF201403018) and the Heilongjiang Postdoctoral Fund (LBH-Z13059).Notes and references
- S. A. Mousavi Shaegh, N.-T. Nguyen, S. M. Mousavi Ehteshami and S. H. Chan, A membraneless hydrogen peroxide fuel cell using prussian blue as cathode material, Energy Environ. Sci., 2012, 5, 8225 CAS.
- Y. Yamada, S. Yoshida, T. Honda and S. Fukuzumi, Protonated iron–phthalocyanine complex used for cathode material of a hydrogen peroxide fuel cell operated under acidic conditions, Energy Environ. Sci., 2011, 4, 2822 CAS.
- Y. Yamada, Y. Fukunishi, S.-i. Yamazaki and S. Fukuzumi, Hydrogen peroxide as sustainable fuel: electrocatalysts for production with a solar cell and decomposition with a fuel cell, Chem. Commun., 2010, 46, 7334 RSC.
- S.-i. Yamazaki, Z. Siroma, H. Senoh, T. Ioroi, N. Fujiwara and K. Yasuda, A fuel cell with selective electrocatalysts using hydrogen peroxide as both an electron acceptor and a fuel, J. Power Sources, 2008, 178, 20 CrossRef CAS PubMed.
- F. Yang, K. Cheng, T. Wu, Y. Zhang, J. Yin, G. Wang and D. Cao, Dendritic palladium decorated with gold by potential pulse electrodeposition: Enhanced electrocatalytic activity for H2O2 electroreduction and electrooxidation, Electrochim. Acta, 2013, 99, 54 CrossRef CAS PubMed.
- F. Yang, K. Cheng, X. Xiao, J. Yin, G. Wang and D. Cao, Nickel and cobalt electrodeposited on carbon fiber cloth as the anode of direct hydrogen peroxide fuel cell, J. Power Sources, 2014, 245, 89 CrossRef CAS PubMed.
- F. Yang, K. Cheng, X. Xue, J. Yin, G. Wang and D. Cao, Three-dimensional porous Ni film electrodeposited on Ni foam: High performance and low-cost catalytic electrode for H2O2 electrooxidation in KOH solution, Electrochim. Acta, 2013, 107, 194 CrossRef CAS PubMed.
- S. B. Hall, E. A. Khudaish and A. L. Hart, Electrochemical oxidation of hydrogen peroxide at platinum electrodes. Part 1. An adsorption-controlled mechanism, Electrochim. Acta, 1997, 43, 579 CrossRef.
- S. B. Hall, E. A. Khudaish and A. L. Hart, Electrochemical oxidation of hydrogen peroxide at platinum electrodes. Part II: effect of potential, Electrochim. Acta, 1998, 43, 2015 CrossRef CAS.
- S. B. Hall, E. A. Khudaish and A. L. Hart, Electrochemical oxidation of hydrogen peroxide at platinum electrodes. Part III: Effect of temperature, Electrochim. Acta, 1999, 44, 2455 CrossRef CAS.
- M. Gerlache, Z. Senturk, G. Quarin and J.-M. Kauffmann, Electrochemical Behavior of H2O2 on Gold, Electroanalysis, 1997, 9, 1088 CrossRef CAS.
- M. Honda, T. Kodera and H. Kita, On the electrochemical behavior of H2O2 at Ag in alkaline solution, Electrochim. Acta, 1983, 28, 727 CrossRef CAS.
- A. Aytaç, M. Gürbüz and A. E. Sanli, Electrooxidation of hydrogen peroxide and sodium borohydride on Ni deposited carbon fiber electrode for alkaline fuel cells, Int. J. Hydrogen Energy, 2011, 36, 10013 CrossRef PubMed.
- C. Iwakura, Y. Fukumoto, H. Inoue, S. Ohashi, S. Kobayashi, H. Tada and M. Abe, Electrochemical characterization of various metal foils as a current collector of positive electrode for rechargeable lithium batteries, J. Power Sources, 1997, 68, 301 CrossRef CAS.
- H.-C. Wu, E. Lee, N.-L. Wu and T. R. Jow, Effects of current collectors on power performance of Li4Ti5O12 anode for Li-ion Battery, J. Power Sources, 2012, 197, 301 CrossRef CAS PubMed.
- S.-L. Sin, L.-L. Teo, K.-S. Tan and C.-Y. Chan, ESCA and TOF-SIMS study on oxidised and reduced polypyrrole-poly(4-styrenesulfonate) free-standing films synthesized on Ti electrodes, Electrochem. Commun., 2000, 2, 685 CrossRef CAS.
- G. Wang and Y. Lin, Performance characteristics of dye-sensitized solar cells with counter electrode based on NiP-plated glass and titanium plate, Curr. Appl. Phys., 2009, 9, 1005 CrossRef PubMed.
- G. Fonder, C. Volcke, B. Csoka, J. Delhalle and Z. Mekhalif, Electrochemical and spectroscopic study of C12H25X molecules adsorption on copper sheets, X (–SH, –S–S–, –SeH and –Se–Se–), Electrochim. Acta, 2010, 55, 1557 CrossRef CAS PubMed.
- X. H. Huang, X. H. Xia, Y. F. Yuan and F. Zhou, Porous ZnO nanosheets grown on copper substrates as anodes for lithium ion Batteries, Electrochim. Acta, 2011, 56, 4960 CrossRef CAS PubMed.
- A. Perrard, L. Retailleau, R. Berjoan and J.-P. Joly, Liquid phase oxidation kinetics of an excellulose activated carbon cloth by NaOCl, Carbon, 2012, 50, 2226 CrossRef CAS PubMed.
- M.-C. Tsai, T.-K. Yeh, Z.-Y. Juang and C.-H. Tsai, Physical and electrochemical characterization of platinum and platinum–ruthenium treated carbon nanotubes directly grown on carbon cloth, Carbon, 2007, 45, 383 CrossRef CAS PubMed.
- Q. Si, M. Kawakubo, M. Matsui, T. Horiba, O. Yamamoto, Y. Takeda, N. Seki and N. Imanishi, Silicon-carbon composite dispersed in a carbon paper substrate for solid polymer lithium-ion batteries, J. Power Sources, 2014, 248, 1275 CrossRef CAS PubMed.
- N. Feng, D. Hu, P. Wang, X. Sun, X. Li and D. He, Phys. Chem. Chem. Phys., 2013, 15, 9924 RSC.
- K. Ji, C. Xu, H. Zhao and Z. Dai, Electrodeposited lead-foam grids on copper-foam substrates as positive current collectors for lead-acid batteries, J. Power Sources, 2014, 248, 307 CrossRef CAS PubMed.
- X. Niu, Y. Li, J. Tang, Y. Hu, H. Zhao and M. Lan, Electrochemical sensing interfaces with tunable porosity for nonenzymatic glucose detection: A Cu foam case, Biosens. Bioelectron., 2014, 51, 22 CrossRef CAS PubMed.
- Z. Bi, M. P. Paranthaman, P. A. Menchhofer, R. R. Dehoff, C. A. Bridges, M. Chi, B. Guo, X.-G. Sun and S. Dai, Self-organized amorphous TiO2 nanotube arrays on porous Ti foam for rechargeable lithium and sodium ion batteries, J. Power Sources, 2013, 222, 461 CrossRef CAS PubMed.
- W. Chen, R. B. Rakhi, L. Hu, X. Xie, Y. Cui and H. N. Alshareef, High-performance nanostructured supercapacitors on a sponge, Nano Lett., 2011, 11, 5165 CrossRef CAS PubMed.
- W. Chen, R. B. Rakhi and H. N. Alshareef, High energy density supercapacitors using macroporous kitchen sponges, J. Mater. Chem., 2012, 22, 14394 RSC.
- L. Fu, G. Qi, R. Sahore, R. Sougrat, F. J. DiSalvo and E. P. Giannelis, Phys. Chem. Chem. Phys., 2013, 15, 19134–19137 RSC.
- L. Hu, H. S. Kim, J.-Y. Lee, P. Peumans and Y. Cui, Scalable coating and properties of transparent, flexible, silver nanowire electrodes, ACS Nano, 2010, 4, 2955 CrossRef CAS PubMed.
- Q. Cheng, Z. Song, T. Ma, B. B. Smith, R. Tang, H. Yu, H. Jiang and C. K. Chan, Nano Lett., 2013, 13, 4969 CrossRef CAS PubMed.
- L. Hu, M. Pasta, F. L. Mantia, L. Cui, S. Jeong, H. D. Deshazer, J. W. Choi, S. M. Han and Y. Cui, Nano Lett., 2010, 10, 708 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |