Synthesis and characterization of the thermodynamic and electrochemical properties of tetra-alkyl phosphonium oxalate ionic liquids†
Mauricio Quiroz-Guzmana,
Daniel P. Fagnant Jr.a,
Xiao-Yan Chenb,
Chaojun Shia,
Joan F. Brennecke*a,
George S. Goff*b and
Wolfgang Runde*c
aDepartment of Chemical and Biomolecular Engineering, University of Notre Dame, 182 Fitzpatrick Hall, Notre Dame, IN 46556, USA. E-mail: jfb@nd.edu; mquirozg@nd.edu; Fax: +1-574-631-8366; Tel: +1-574-631-5847
bChemistry Division and Science Program Office, Los Alamos National Laboratory, Los Alamos, New Mexico 87545, USA. E-mail: georgeg@lanl.gov
cScience Program Office, Los Alamos National Laboratory, Los Alamos, New Mexico 87545, USA. E-mail: runde@lanl.gov
First published on 12th March 2014
Abstract
Ionic liquids (ILs) are attractive alternatives to water, high temperature molten salts or conventional organic solvents for electrodeposition of technologically important metals. Designing ILs containing functional groups specifically targeted to interact with or even chelate metals, such as carboxylate, may increase the metal solubility and affect thermodynamic and electrochemical properties. This paper reports the synthesis and characterization of three ionic liquids comprised of one or two tetra-alkyl phosphonium cations paired with a hydrogen oxalate or oxalate anion, with the general formula [(PRRRR′)x][C2O4H2−x], R = 4,6 and R′ = 4, 14. These compounds were characterized by 1H, 13C, 31P NMR, and Raman spectroscopy. Electrochemical windows, electrical conductivities, densities and viscosities were also determined experimentally. Thermal gravimetric analysis (TGA) revealed decomposition temperatures above 200 °C and differential scanning calorimetry (DSC) analysis revealed that the two [(P66614)x][C2O4H2−x] salts have similar glass transition temperatures while [(P4444)2][C2O4] has a melting point above room temperature. Raman and powder X-ray diffraction spectroscopy confirm that Nd2O3 dissolves and complexes with [P66614][C2O4].
Introduction
Ionic liquids (ILs) have become very attractive alternatives to common molecular solvents in many research areas, including synthesis,1 catalysis,2 liquid–liquid extraction of various solutes including metal ions,3–7 and electrochemistry, where they are often used as electrolytes in batteries and photovoltaic devices.6,7 Insignificant vapor pressure, large liquidus range, high thermal stability, high electrical conductivity, wide electrochemical windows, and selective substrate solubility are some of the characteristics that make ILs suitable agents for these and many other potential applications.7,8One particularly interesting potential application is the use of ILs as substitutes for high temperature molten salts in the recovery of actinides and pyroprocessing of used nuclear fuel rods.9–11 Typically high-temperature molten inorganic salts act as an electrolytic medium at process temperatures of 700 K or higher, depending on the composition of the molten electrolyte.12,13 The lower melting temperature, lower operation temperature, and decreased corrosivity of ILs may reduce the operating costs for such processes. Whereas the use of ILs as media for electrodeposition of non-ferrous metals has been explored for some time,14–17 the synthesis of new ILs specifically tailored for this task may help to move this technology towards commercialization.
Towards this end, we have designed a set of phosphonium oxalate ILs where we seek to emphasize three desirable attributes: (i) thermal stability: phosphonium-like cationic moieties are known for their great chemical and thermal stability and relatively low toxicity compared to their ammonium-based analogues,18–20 (ii) electrochemical activity: alkyl phosphonium-based ILs display wide electrochemical windows and good conductivities,18,20–23 and in some cases better electrochemical properties than their alkyl ammonium counterparts,24 (iii) chelating ability: carboxylate-based molecules have long been recognized as excellent precipitating/chelating agents for f-block elements in a variety of media.25–33
Alkyl phosphonium oxalates have been previously cited in the literature, but merely as a component in a complex mixture.34 Specifically, they have been used as phase transfer catalysts in the production of aromatic carbonates,35 and as functionalizing agents to modify ion exchange resins for the production of butenyl acetate.36 In neither case were they isolated, characterized nor even identified as an IL. A similar situation exists for the analogous alkyl ammonium oxalates.37–40
By developing three new phosphonium oxalate ILs we attempted to achieve high thermal stability and conductivity, suitable electrochemical windows, and a potential for selective solubilization of metals and metal oxides promoted by the oxalate chelating ion.27,41,42 These ILs could offer an alternative electrolyte media to the molten salt technologies presently used in the electroplating industry.
Results and discussion
The synthesis of the phosphonium oxalate ILs was accomplished by reacting oxalic acid with the desired molar ratio of tetra-alkyl phosphonium hydroxide (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 or 1
1 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2), depending on the degree of protonation intended in the oxalate core, to produce tetra-alkyl phosphonium carboxyformate [(PRRRR′)][HOx] or bis tetra-alkyl phosphonium oxalate [(PRRRR′)2][Ox] ILs, respectively (Fig. 1).
2), depending on the degree of protonation intended in the oxalate core, to produce tetra-alkyl phosphonium carboxyformate [(PRRRR′)][HOx] or bis tetra-alkyl phosphonium oxalate [(PRRRR′)2][Ox] ILs, respectively (Fig. 1).
Tetra-alkyl phosphonium hydroxide was prepared via ion exchange between the respective alkyl phosphonium bromide and amberlite hydroxide anion exchange resin. All the reactions were carried out in methanol using common wet bench chemistry procedures.
NMR analysis
The 1H NMR spectra of the three phosphonium oxalate ILs show a similar pattern of three sets of multiplets: the first multiplet in the range of 2.19–2.25 ppm accounts for the 8 α-hydrogens adjacent to the phosphorous atom, a second multiplet between 1.23–1.45 ppm comprises all the methylene protons and, a third multiplet in the range of 0.85–0.92 ppm accounts for all the terminal methyl groups in each phosphonium cation. [P66614][HOx] is the only IL that displays a hydroxyl signal at 11.92 ppm. The 13C NMR spectra seem cumbersome due to the long aliphatic chains of the phosphonium cation, but they are very diagnostic, as they all show the presence of a carbonyl carbon between 164.3 and 174.7 ppm from the oxalic acid. Since only one carbonyl carbon is observed in all the cases it is assumed that the anions in all three salts display C2 symmetry, even in [P66614][HOx], but in this case a degeneracy of the carbonyl carbon might be a more reasonable explanation. 31P NMR spectra revealed a single peak for each compound between 34.7–34.9 ppm. Integration of these singlets shows 97%+ purity of phosphorous for each IL prepared. It is important to mention that the starting [P66614][Br] was ≥95% purity. The peaks observed around 38 and 47 ppm could be isoalkyl impurities present in the phosphonium bromides and oxidation products of O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) PR3, respectively.43 This assumption is made based on the possible products that would match the observed chemical shifts. All NMR spectra are shown in the ESI.†
PR3, respectively.43 This assumption is made based on the possible products that would match the observed chemical shifts. All NMR spectra are shown in the ESI.†
Volatiles in the three ILs were removed under reduced pressure and mild heating (60 °C) over the period of 3 weeks. [(P66614)2][Ox] was obtained as a yellow highly viscous semisolid, [P66614][HOx] as a yellow liquid and [(P4444)2][Ox] as a yellow solid. Despite prolonged heating and vacuuming, some methanol and water impurities were detected by 1H NMR spectroscopy. [(P66614)2][Ox] contained both water at 3.56 ppm and methanol at 3.13 ppm; [P66614][HOx] and [(P4444)2][Ox] contained only water, observed at 3.57 and 3.60 ppm, respectively. Chemical shifts for the residual water peaks deviated somewhat from the expected literature value of 3.33 ppm in DMSO-d6.44 However, this is consistent with what we have observed in practice for P66614-based ILs doped with water, which have shown water chemical shifts at 4.0 ppm at large water concentrations. This is reasonable because the ILs can interact and form intermolecular hydrogen bonds with both water and methanol (and with each other), which could affect the observed chemical shifts by moving the resonance signal of the hydrogen bonding proton to lower field.45,46
Raman analysis
The Raman spectra for the three phosphonium oxalate ILs are shown in Fig. 2. The water contents were 1.29, 0.28 and 0.21 wt% for [(P4444)2][Ox], [(P66614)2][Ox] and [P66614][HOx]. The asymmetrically alkylated phosphonium cations show a distinctive feature over their symmetrically alkylated analog. In the case of [(P4444)2][Ox], the strongest Raman signals are located in the CH stretching region (2760–3050 cm−1), assigned to the CH stretching vibrations of the alkyl chains.47 The Raman band at 1450 cm−1 is attributed to the symmetric stretching mode ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and the Raman band at 1312 cm−1 is attributed to the carboxylic bending δ(C–O) modes.48 The symmetric stretching mode ν(C–O) is observed at 1101 cm−1. The peak at 1049 cm−1 is assigned as the ν(C–C) stretching of the alkyl chain.47 In [(P66614)2][Ox] and [P66614][HOx], this single peak splits into double peaks at 1073 and 1063 cm−1 which can be attributed to the two different alkyl chain lengths. The weak peak at 1012 and 967 cm−1 can be assigned as a CH3 rocking mode.49 The peak at 887 with a shoulder at 866 cm−1 is due to the ν(C–C) stretching. The symmetric P–C stretching is observed as a single vibration at 673 cm−1.49 The assignments are summarized in Table 1.
O) and the Raman band at 1312 cm−1 is attributed to the carboxylic bending δ(C–O) modes.48 The symmetric stretching mode ν(C–O) is observed at 1101 cm−1. The peak at 1049 cm−1 is assigned as the ν(C–C) stretching of the alkyl chain.47 In [(P66614)2][Ox] and [P66614][HOx], this single peak splits into double peaks at 1073 and 1063 cm−1 which can be attributed to the two different alkyl chain lengths. The weak peak at 1012 and 967 cm−1 can be assigned as a CH3 rocking mode.49 The peak at 887 with a shoulder at 866 cm−1 is due to the ν(C–C) stretching. The symmetric P–C stretching is observed as a single vibration at 673 cm−1.49 The assignments are summarized in Table 1.
| Assignment | [(P4444)2][Ox] (cm−1) | [(P66614)2][Ox] (cm−1) | [P66614][HOx] (cm−1) |
|---|---|---|---|
| ν(CH) | 2760–3050 | 2760–3050 | 2760–3050 |
ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) O) |
1450 | 1437 | 1440 |
| δ(C–O) | 1312 | 1303 | 1305 |
| ν(C–O) | 1101 | 1113 | 1112 |
| ν(C–C) | 1049 | 1073, 1063 | 1073, 1063 |
| ρ(CH3) | 1012, 967 | 1016, 964 | 1009, 966 |
| ν(C–C) | 887, 866 | 886, 845 | 888, 846 |
| ν(P–C) | 671 | 673 | 668 |
Thermal gravimetric analysis and differential scanning calorimetry
A thermal gravimetric analyzer (TGA) and a differential scanning calorimeter (DSC) were used to determine the decomposition temperature (Tonset), glass transition temperature (Tg) and melting temperature (Tm) of the phosphonium oxalate ILs. In the TGA all samples were heated at 110 °C for 45 min before increasing the temperature in order to eliminate any remaining volatile impurities. The following weight losses were observed during this stage: 0.5 wt%; for [P66614][HOx], 1.0 wt% for [(P66614)2][Ox], and 2.4 wt% for [(P4444)2][Ox]. This is consistent with some solvent remaining in the samples, as indicated by 1H NMR spectroscopy. Then the temperature was increased at a constant ramp rate of 10 °C min−1 until the temperature reached 500 °C. The important descriptor of thermal stability is the decomposition onset temperature (Tonset), which is defined as the intersection of the baseline weight and the tangent of the weight vs. temperature. Generally, the decomposition temperature of a given ionic liquid depends on the intrinsic stability that anions and cations may possess,19 and also on the decomposition mechanisms that might arise from interactions between the constituent cations and anions, with dealkylation and nucleophilic substitution being the most important.50,51 The TGA time course scans of [(P66614)2][Ox], [P66614][HOx] and [(P4444)2][Ox], given in the ESI,† show that all three ILs have two inflection points, indicating two decomposition mechanisms. The Tonset values for [(P4444)2][Ox], [(P66614)2][Ox] and [P66614][HOx] are 236 °C, 281 °C and 291 °C, respectively. The majority of the mass loss for [(P4444)2][Ox] comes from the first ‘step’, as seen in ESI.† As a result, the Tonset was determined by the first inflection point. Conversely, most of the mass loss for [(P66614)2][Ox] and [P66614][HOx] come from the second ‘step’, so their Tonset values were determined by the second inflection points. This may explain the apparently higher thermal stability of the [P66614] salts. All in all, Tonset data showed that all three ILs have decent thermal stability.The melting and glass transition temperatures were determined by DSC. The melting point (Tm) is the onset of an endothermic peak on heating the sample from the solid state to the liquid state. The glass transition temperature (Tg) is the midpoint of a small heat capacity change on heating from amorphous glass state to a liquid state. This phenomenon is commonly observed for polymers and other amorphous materials. DSC scans (shown in ESI†) of [(P66614)2][Ox] and [P66614][HOx] show that they have similar glass transition temperatures of −79 and −74 °C, respectively. [(P4444)2][Ox] does not have a glass transition temperature. Rather, the DSC shows two small endothermic peaks that are most likely melting points(Tm = −18/93 °C). The two melting points may be due to polymorphism or the persistent presence of (i.e., solvent) impurities.52
Viscosity and conductivity
[(P66614)2][Ox] is a highly viscous semi-solid and [(P4444)2][Ox] a solid at room temperature so the measurement of their viscosities and conductivities was not possible. By contrast, [P66614][HOx] is a liquid at room temperature and its physicochemical properties are presented in Table 2 from 10 °C to 70 °C. As expected, the observed density has a linear relationship with temperature. The initial density of [P66614][HOx] is less than 1 g cm−3 and decreases with increasing temperature. The low density is consistent with the hydrocarbon nature of the long alkyl chains on the cation. As shown in Table 2, viscosity decreases rapidly with increasing temperature, with the viscosity of [P66614][HOx] at 10 °C being two orders of magnitude higher than that at 70 °C. Chemical structure might also play an important role in the observed changes in viscosities: smaller and symmetric phosphonium cations will permit easier stacking and therefore facilitate the formation of a quasi-solid state, as is the case with [(P4444)2][Ox]. The strong hydrogen-bonding ability of the HOx-anion may be the main contribution to the relatively high viscosity observed for [P66614][HOx] in the temperature range studied. The oxalate moiety is not alien to the ionic liquid literature; tetra-alkyl phosphonium bis(oxalato)borates [PRRRR′][BOB] and, specifically [P66614][BOB], have been studied given their potential use as battery electrolytes.53 [P66614][BOB] has a viscosity lower (615 cP, 20 °C) than that of [P66614][HOx] (1720 cP, 20 °C), the least viscous member of the family of tetra-alkyl phosphonium oxalate ionic liquids presented in this paper.53,54 Viscosity is a property dependent on electrostatic, van der Waals, and hydrogen bonding interactions, as well as others factors such as molecular weight, molecular geometry and charge distribution.1,55,56 The lower viscosity of [P66614][BOB] compared to the tetra-alkyl phosphonium oxalates may be attributable to the size of the larger [BOB]− anion which allows a more disperse charge distribution compared to the smaller oxalate anion. Additionally the mutually perpendicular arrangement of the oxalates moieties in the [BOB]− anion57 may hinder molecular stacking allowing better fluidity at the macroscopic level when compared to the flat oxalate anion in the ILs described here. Conductivity of [P66614][HOx] was measured over the temperature range of 10–70 °C, as well. Overall, [P66614][HOx] exhibits relatively low conductivity because of its high viscosity.| Temp. (°C) | Density (g cm−3) | Viscosity (cP) | Conductivity (mS cm−1) |
|---|---|---|---|
| 10 | 0.94054 | 4470 | 4.01 × 10−3 |
| 20 | 0.93388 | 1720 | 9.99 × 10−3 |
| 25 | 0.93044 | 1110 | 1.52 × 10−2 |
| 30 | 0.92714 | 769 | 2.19 × 10−2 |
| 40 | 0.92076 | 380 | 4.32 × 10−2 |
| 50 | 0.91438 | 207 | 7.86 × 10−2 |
| 60 | 0.90814 | 125 | 1.34 × 10−1 |
| 70 | 0.90191 | 80 | 2.14 × 10−1 |
Fig. 3 displays the Walden plot of [P66614][HOx] at different temperatures. The solid line that runs from corner to corner is the ideal line, and the dashed line is one log unit below the ideal line. As shown in Fig. 3, 1-hexyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ([Hmim][Tf2N]) lies relatively close to the ideal line, indicating that it is a relatively good electrolyte. Trihexyltetradecyl phosphonium bis(trifluoromethyl-sulfonyl)imide ([P66614][Tf2N]) and [P66614][HOx] are located progressively further from the ideal line, indicating that they have lower ‘ionicity’ and are not as good of electrolytes as [Hmim][Tf2N]. [P66614][HOx] presents the lowest ionicity of the three ILs shown, exhibiting only 10% of the ideal molar conductivity. The slope of the [P66614][HOx] molar conductivity in the temperature range of 10–70 °C is around one, which indicates that the degree of dissociation of [P66614][HOx] is independent of temperature.
 | ||
| Fig. 3 Walden plot for [P66614][HOx] at different temperatures compared to values from the literature for [P66614][Tf2N]18 and [Hmim][Tf2N].58 | ||
Electrochemical windows
The electrochemical window (EW) of an IL is an indication of the resistance of the cation to reduction and the anion to oxidation. The electrochemical window of [P66614][HOx] was determined by cyclic voltammetry at 40 °C on Pt and GC (diameter 1 mm) working electrodes vs. a Ag wire quasi-reference electrode. This was the minimum temperature at which a well-behaved cyclic voltammogram could be obtained. The scan rate of the cyclic voltammetry was 100 mV s−1 with the limits of the electrochemical window determined to be when the current density reached 1.0 mA cm−2. With a GC working electrode, the cathodic potential limit (Epc) is −3.6 V and the anodic potential limit (Epa) is 2.1 V (Fig. 4).Conventionally, the electrochemical window is given by EW = Epa − Epc = 5.7 V. This result is comparable to the published data for electrochemical windows of non-functionalized phosphonium ILs, with GC working electrodes (5.23–6.9 V).20,23,59,60 When using the Pt working electrode, Epc shrinks to −1.7 V, and Epa shrinks to 1.7 V, leading to a much smaller electrochemical window of 3.4 V. The Pt working electrode has a significantly lower overpotential for proton reduction compared to GC, thus causing a smaller electrochemical window for [P66614][HOx]. In comparison to the protic carboxyl-functionalized phosphonium IL, 1-carboxy-N,N,N-trimethyl phosphonium bis(trifluoromethyl-sulfonyl)imide, which has an EW of 3.12 V at 100 °C, [P66614][HOx] has a considerably wider EW with an improved cathodic stability.61 Since the measurement of EWs is somewhat subjective, it is difficult to compare different ILs due to the variabilities in working electrodes, reference systems, experimental conditions and threshold values. Due to the highly viscous and even solid nature of the two other phosphonium oxalate ILs, adequate cyclic voltammograms could not be obtained even at temperatures as high as 145 °C. It should also be noted that all three ILs give off white fumes when heated at elevated temperatures in the presence of air, indicating these ILs have lower thermal stability than indicated by the previous thermal analysis performed under N2. [P66614][HOx] showed the highest stability in air, with only minimal fuming observed at 40 °C.
Nd(III)–[P66614][HOx] complexation
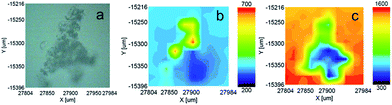
[P66614][HOx] exhibits the highest thermal stability and the lowest viscosity of the three ILs synthesized in this work making it a suitable candidate for complexation studies. We used Nd2O3 as an example. Solid Nd2O3 was dissolved at 80 °C into a water saturated [P66614][HOx] solution, resulting in the overnight precipitation of a light blue solid shown in Fig. 5a.Raman spectroscopy was used to determine the presence of a Nd–oxalate complex since Morris and Hobart showed that lanthanide oxalates are readily identifiable by peaks at ca. 1480 cm−1, 920 cm−1 and 500 cm−1.62 Raman spectra of [P66614][HOx] before and after the addition of Nd2O3, as well as of the blue precipitate are shown in ESI.† The spectrum of the blue solid precipitate has peaks at 1480 cm−1, 920 cm−1 and 500 cm−1, confirming the presence of a neodymium oxalate complex. These peaks were not present in the Raman spectra of pure [P66614][HOx] or in the supernatant IL liquid after Nd2O3 dissolution. We believe this is due to the limited solubility of the Nd(III)–oxalate complex in the IL. A 2D map of the most intense Nd(III)-oxalate peak at 1474 cm−1 can be seen in Fig. 5b. Orange represents the highest intensity and blue the lowest intensity. Clearly, Nd(III)-oxalate is present in the upper part of the precipitated sample. Fig. 5c shows a 2D map of the Raman intensity for 2883 cm−1, which is characteristic of C–C stretches in the alkyl chains of the [P66614] cation. The IL cation is definitely present in the liquid on the microscope slide surrounding the solid particles. It is present in lower concentrations in the area where Nd(III)-oxalate is present, and almost entirely absent in the lower half of the precipitated solid particles. We attribute the lack of a Raman signal in the lower half of the solid for both the Nd(III)–oxalate complex (Fig. 5b) and [P66614] cation (Fig. 5c) to unreacted Nd2O3, which is not Raman active at these wavenumbers. In addition, the moderate intensity of the [P66614] cation (green in Fig. 5c) in the same location as the high concentration of the Nd(III)-oxalate signal (yellow in Fig. 5b) indicates that the Nd(III)–oxalate complex may contain [P66614] counteranions after precipitation. Additional Raman and powder X-ray diffraction characterization can be found in the ESI.†
Conclusions
Three different tetra-alkyl phosphonium oxalate ionic liquids were synthesized and characterized through 1H, 13C and 31P NMR spectroscopy. [(P4444)2][Ox] displayed two melting points: Tm = −18/93 °C, [(P66614)2][Ox] was a highly viscous semi-solid. By contrast, [P66614][HOx] was a clear, relatively free-flowing liquid. All three ILs showed good thermal stability. The [P66614][HOx] was fully characterized in terms of density, viscosity, electrical conductivity and electrochemical window, and was shown to complex with neodymium. Due to the high viscosity and low electrical conductivity at room temperature, it is likely that use of these ILs for electroplating would be more effective at elevated temperatures.Experimental
General
Unless otherwise noted, all procedures were carried out using common wet bench procedures. Ion exchange resins Amberlite® IRN78 and Dowex SBR LC NG hydroxide form were purchased from Aldrich and used interchangeably. The resin beads were washed three times with methanol prior to their use in every ion exchange stage. Methanol ACS grade, 99.8% (VWR), oxalic acid dihydrate, 98% (VWR), trihexyl(tetradecyl)phosphonium bromide, ≥95% ([P66614][Br]) (Aldrich), tetrabutyl phosphonium hydroxide ([P4444][OH]) 40% w/w aqueous solution (Hokko Chem. Ind.) and neodymium oxide, ≥ 99.9% metal basis (Nd2O3) (Sigma Aldrich), were used without any further purification. Anhydrous deuterated dimethyl sulfoxide (DMSO-d6) was obtained from Cambridge Isotope Laboratories. Routine NMR spectra were measured on a Varian VXR-300 for 31P or a Varian INOVA-500 spectrometer for 1H and 13C{1H}. Chemical shifts for 1H and 13C{1H} spectra are reported in ppm downfield of tetramethylsilane, using the known chemical shifts of the solvent residual signals. Raman spectra were collected in a Thermo Scientific DXR SmartRaman spectrometer with a 780 nm excitation laser. Thermal gravimetric analyses were recorded in a Mettler Toledo TGA 851e where products were first heated from 25 °C to 110 °C at a rate of 20 °C min−1, maintained at 110 °C for 45 minutes and then heated from 110 °C to 500 °C at a rate of 10 °C min−1 (dynamic decomposition) under a N2 atmosphere.The melting and glass transition temperatures were determined in a Mettler Toledo DSC822e differential scanning calorimeter at a ramp rate of 10 °C min−1 from −120 to 110 °C under N2. Densities were measured in a DMA 4500 Anton Paar oscillating U-tube densitometer at atmospheric pressure with automatic correction for the viscosity. The uncertainty of density measurements is ±5 × 10−5 g cm−3. Two integrated Pt 100 thermometers provided precision (±0.01 K) in internal temperature control. The viscosity of [P66614][HOx] was determined with an ATS Rheosystems Viscoanalyzer containing the ETC-3 Joule Thomson effect temperature cell with an operating temperature range from −10 to 400 °C. The uncertainty in the measured viscosity is ±5% when the viscosity is above 100 cP. The temperature dependence of the conductivity of [P66614][HOx] was measured with an electrochemical impedance spectroscopy (EIS) system, equipped with a Solartron SI 1260 Impedance/Gain-phase analyzer and a Solartron 1287 electrochemical interface. The conductivity sample cell constant was calibrated with KCl standard solution. IL samples were loaded inside a glove box with N2 atmosphere and thermally equilibrated around two hours before each measurement. Electrochemical windows were recorded using a computer-controlled Solartron 273A Potentiostat/Galvanostat (EG&G, Princeton Applied Research). All the electrodes were purchased from Cypress Systems. The counter electrode was a Pt mesh and the reference electrode was a silver wire. Water content was measured by Karl-Fisher titration using an 852 Titrando (Metrohm USA, Inc.). The water content of [P66614][HOx] used for electrochemical window measurements was 0.21 wt%.
1.57 grams of [P66614][HOx], 0.53 grams of deionized water and 0.0036 grams of Nd2O3 were mixed in a sealed container and heated at 80 °C overnight. A light blue solid precipitated from the mixture. Raman spectra were collected on a JASCO NRS-5100 micro Raman spectrometer with a 532 nm excitation laser and an estimated resolution of 7 cm−1. 2D Raman mapping experiments were conducted on a 10 × 10 grid across the sample image. Powder X-ray diffraction experiments were collected on a Bruker Davinci D8.
Synthesis
Acknowledgements
The authors gratefully acknowledge the Los Alamos Laboratory Directed Research and Development Program and the G. T. Seaborg Institute for Transactinium Science at Los Alamos National Laboratory for financial support during this project.References
- P. Wasserscheid and W. Keim, Angew. Chem., Int. Ed., 2000, 39, 3772–3789 CrossRef CAS.
- T. Welton, Chem. Rev., 1999, 99, 2071–2083 CrossRef CAS PubMed.
- K. Nakashima, F. Kubota, T. Maruyama and M. Goto, Ind. Eng. Chem. Res., 2005, 44, 4368–4372 CrossRef CAS.
- L. Zhu, L. Guo, Z. Zhang, J. Chen and S. Zhang, Sci. China: Chem., 2012, 55, 1479–1487 CrossRef CAS.
- J.-H. Olivier, F. Camerel and R. Ziessel, Chem.–Eur. J., 2011, 17, 9113–9122 CrossRef CAS PubMed.
- K. Binnemans, Chem. Rev., 2007, 107, 2592–2614 CrossRef CAS PubMed.
- M. Freemantle, An introduction to ionic liquids, The Royal Society of Chemistry, Cambridge, UK, 2009 Search PubMed.
- J. F. Brennecke, C. M. Gordon, D. M. Haddleton, J. H. Davis, M. J. Earle, F. Endres, C. Hardacre, A. Dölle, C. Hilgers, J. Holbrey, K. R. Seddon, U. Kragl, P. C. Trulove, H. C. de Long, P. Wasserscheid, H. Olivier-Bourbigou, T. Welton, R. D. Rogers and J. S. Wilkes, Ionic Liquids in Synthesis, Federal Republic of Germany, 2nd edn, 2002, vol. 7 Search PubMed.
- J. C. Rao, K. A. Venkatesan, K. Nagarajan, T. G. Srinivasan and V. P. R. Rao, J. Nucl. Mater., 2011, 408, 25–29 CrossRef.
- K. Takao, T. J. Bell and Y. Ikeda, Inorg. Chem., 2013, 52, 3459–3472 CrossRef CAS PubMed.
- K. A. Venkatesan, T. G. Srinivasan and P. R. V. Rao, J. Nucl. Radiochem. Sci., 2009, 10, 1–6 Search PubMed.
- W. H. Kruesi and D. J. Fray, Metall. Mater. Trans. B, 1993, 24B, 605–615 CrossRef CAS.
- P. Giridhar, K. a. Venkatesan, T. G. Srinivasan and P. R. V. Rao, Electrochim. Acta, 2007, 52, 3006–3012 CrossRef CAS.
- J. a. Whitehead, G. a. Lawrance and A. McCluskey, Green Chem., 2004, 6, 313–315 RSC.
- F. Endres, ChemPhysChem, 2002, 3, 144–154 CrossRef CAS.
- Y.-S. Liu and G.-B. Pan, in Ionic Liquids: Applications and Perspectives, ed. A. Kokorin, InTech, India, 2011, pp. 627–642 Search PubMed.
- Y. Katayama, in Electrochemical Aspects of Ionic Liquids, ed. H. Ohno, John Wiley & Sons, Inc., Hoboken, NJ, USA, 2nd edn, 2011, pp. 129–155 Search PubMed.
- K. J. Fraser and D. R. MacFarlane, Aust. J. Chem., 2009, 62, 309–321 CrossRef CAS.
- J. Kagimoto, K. Fukumoto and H. Ohno, Chem. Commun., 2006, 2254–2256 RSC.
- K. Tsunashima and M. Sugiya, Electrochem. Commun., 2007, 9, 2353–2358 CrossRef CAS.
- M. Matsumiya, S. Suda, K. Tsunashima, M. Sugiya, S. Kishioka and H. Matsuura, J. Electroanal. Chem., 2008, 622, 129–135 CrossRef CAS.
- K. Tsunashima, A. Kawabata, M. Matsumiya, S. Kodama, R. Enomoto, M. Sugiya and Y. Kunugi, Electrochem. Commun., 2011, 13, 178–181 CrossRef CAS.
- K. Tsunashima, Y. Ono and M. Sugiya, Electrochim. Acta, 2011, 56, 4351–4355 CrossRef CAS.
- J. a. Vega, J. Zhou and P. a. Kohl, J. Electrochem. Soc., 2009, 156, A253–A259 CrossRef CAS.
- M. R. Spirlet and J. R. Rebizant, Acta Crystallogr., 1987, C43, 19–21 CAS.
- D. L. Clark, S. D. Conradson, D. W. Keogh, P. D. Palmer, B. L. Scott and C. D. Tait, Inorg. Chem., 1998, 37, 2893–2899 CrossRef CAS.
- G. Andreev, N. Budantseva, A. Fedoseev and P. Moisy, Inorg. Chem., 2011, 50, 11481–11486 CrossRef CAS PubMed.
- A. V. Vologzhanina, L. B. Serezhkina, N. a. Neklyudova and V. N. Serezhkin, Inorg. Chim. Acta, 2009, 362, 4921–4925 CrossRef CAS.
- S. Govindarajan, K. C. Patil, M. D. Poojary and H. Manohar, Inorg. Chim. Acta, 1986, 120, 103–107 CrossRef CAS.
- N. W. Alcook, J. Chem. Soc., Dalton Trans., 1973, 1614–1616 RSC.
- G. E. Sigmon, J. Ling, D. K. Unruh, L. Moore-Shay, M. Ward, B. Weaver and P. C. Burns, J. Am. Chem. Soc., 2009, 131, 16648–16649 CrossRef CAS PubMed.
- B. Chapelet-Arab, G. Nowogrocki, F. Abraham and S. Grandjean, Radiochim. Acta, 2005, 93, 279–285 CrossRef CAS.
- W. Hummel, I. Puigdomènech, L. Rao and O. Tochiyama, C. R. Chim., 2007, 10, 948–958 CrossRef CAS.
- C. M. Starks, J. Am. Chem. Soc., 1970, 93, 195–199 CrossRef.
- J. E. Hallgren, US Pat. 4201721, 1980.
- T. E. Paxson, US Pat. 4450289, 1984, pp. 58–60.
- C.-T. Yang, Y. Fu, Y.-B. Huang, J. Yi, Q.-X. Guo and L. Liu, Angew. Chem., Int. Ed., 2009, 48, 7398–7401 CrossRef CAS PubMed.
- V. M. Kamhi, US Pat. 5359089, 1994, pp. 4–8.
- Z. Q. Zheng, J. Wang, T. H. Wu and X. P. Zhou, Adv. Synth. Catal., 2007, 349, 1095–1101 CrossRef CAS.
- J. Du Preez and G. Hermanus, US Pat. 7956208 B2, 2011.
- D. A. Mew, H. Krikorian, K. E. Dodson and J. A. Schmitz, Purification of 238Pu Oxide using the Pu Oxalate Process, 2001 Search PubMed.
- W. Runde, L. F. Brodnax, G. Goff, A. C. Bean and B. L. Scott, Inorg. Chem., 2009, 48, 5967–5972 CrossRef CAS PubMed.
- C. R. Hilliard, N. Bhuvanesh, J. a. Gladysz and J. Blümel, Dalton Trans., 2012, 41, 1742–1754 RSC.
- G. R. Fulmer, A. J. M. Miller, N. H. Sherden, H. E. Gottlieb, A. Nudelman, B. M. Stoltz, J. E. Bercaw and K. I. Goldberg, Organometallics, 2010, 29, 2176–2179 CrossRef CAS.
- E. Arunan, G. R. Desiraju, R. a. Klein, J. Sadlej, S. Scheiner, I. Alkorta, D. C. Clary, R. H. Crabtree, J. J. Dannenberg, P. Hobza, H. G. Kjaergaard, A. C. Legon, B. Mennucci and D. J. Nesbitt, Pure Appl. Chem., 2011, 83, 1619–1636 CAS.
- J. E. Del Bene, S. A. Perera and R. J. Bartlett, J. Phys. Chem. A, 1999, 103, 8121–8124 CrossRef CAS.
- A. Kavanagh, R. Copperwhite, M. Oubaha, J. Owens, C. McDonagh, D. Diamond and R. Byrne, J. Mater. Chem., 2011, 21, 8687–8693 RSC.
- R. L. Frost, A. Locke and W. N. Martens, J. Raman Spectrosc., 2008, 39, 901–908 CrossRef CAS.
- J. R. Durig, D. F. Smith, D. A. Barron, R. J. Harlan and H. V. Phan, J. Raman Spectrosc., 1992, 23, 107–124 CrossRef CAS.
- W. Xie, R. Xie, P. Wei-Ping, D. Hunter, B. Koene, T. Loon-Seng and R. Vaia, Chem. Mater., 2002, 15, 4837–4845 CrossRef.
- Y. Chen, Y. Cao, Y. Shi, Z. Xue and T. Mu, Ind. Eng. Chem. Res., 2012, 51, 7418–7427 CrossRef CAS.
- K. Ma, K.-M. Lee, L. Minkova and R. G. Weiss, J. Org. Chem., 2009, 74, 2088–2098 CrossRef CAS PubMed.
- S. I. Lall-Ramnarine, A. Castano, G. Subramaniam, M. F. Thomas and J. F. Wishart, Radiat. Phys. Chem., 2009, 78, 1120–1125 CrossRef CAS.
- F. U. Shah, S. Glavatskih, D. R. MacFarlane, A. Somers, M. Forsyth and O. N. Antzutkin, Phys. Chem. Chem. Phys., 2011, 13, 12865–12873 RSC.
- T. Rüther, T. D. Huynh, J. Huang, A. F. Hollenkamp, E. A. Salter, A. Wierzbicki, K. Mattson, A. Lewis and J. H. Davis, Chem. Mater., 2010, 22, 1038–1045 CrossRef.
- M. Nieuwenhuyzen, K. R. Seddon, F. Teixidor, A. V Puga and C. Viñas, Inorg. Chem., 2009, 48, 889–901 CrossRef CAS PubMed.
- P. Y. Zavalij, S. Yang and M. S. Whittingham, Acta Crystallogr., Sect. B: Struct. Sci., 2003, 59, 753–759 Search PubMed.
- J. A. Widegren, E. M. Saurer, K. N. Marsh and J. W. Magee, J. Chem. Thermodyn., 2005, 37, 569–575 CrossRef CAS.
- S. Kanematsu, K. Matsumoto and R. Hagiwara, Electrochem. Commun., 2009, 11, 1312–1315 CrossRef CAS.
- M. Hayyan, F. S. Mjalli, M. A. Hashim, I. M. AlNashef, X. M. Tan and K. L. Chooi, J. Appl. Sci., 2010, 10, 1176–1180 CrossRef CAS.
- X.-Y. Chen, G. S. Goff, M. Quiroz-Guzman, D. P. Fagnant, J. F. Brennecke, B. L. Scott and W. Runde, Chem. Commun., 2013, 49, 1903–1905 RSC.
- D. E. Morris and D. E. Hobart, J. Raman Spectrosc., 1988, 19, 231–237 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra01467g |
| This journal is © The Royal Society of Chemistry 2014 |