Flash carboxylation: fast lithiation–carboxylation sequence at room temperature in continuous flow†
Bartholomäus Pieber,
Toma Glasnov and
C. O. Kappe*
Christian Doppler Laboratory for Flow Chemistry and Institute of Chemistry, University of Graz, Heinrichstrasse 28, 8010 Graz, Austria. E-mail: oliver.kappe@uni-graz.at; Fax: +43 316 3809840
First published on 3rd March 2014
Abstract
A method for the direct lithiation of terminal alkynes and heterocycles with subsequent carboxylation in a continuous flow format was developed. This method provides carboxylic acids at ambient conditions within less than five seconds with only little excess of the organometallic base and CO2.
Carbon dioxide (CO2) is a highly attractive building block for organic synthesis as it is readily available, extremely cheap, abundant, non-toxic, nonflammable, and is classified as an ideal renewable carbon source.1 Due to its low reactivity a large energy input is required to use the greenhouse gas as reagent for synthetic transformations. Several methods for the utilization of CO2 are well studied and used on laboratory as well as on industrial scales.
The most straightforward strategy to tackle the low reactivity of CO2 is the use of high-energy starting materials such as Grignard or organolithium compounds.1 The latter carbanion equivalents are often highly unstable and are usually prepared at very low temperatures. Furthermore, reactions of these intermediates with electrophiles are difficult to control as a result of their exothermic nature resulting in severe limitations for industrial applications.
Pioneering work by Yoshida on halogen–lithium exchange reactions demonstrated that the generation as well as the use of various organolithium species can be performed at much higher temperatures when a continuous microreactor is used instead of traditional batch macroreactors.2,3 Such “flash chemistry” transformations are typically carried out in the range of (milli-) seconds offering several advantages compared to traditional approaches.4 The use of microreactors in general has become increasingly popular in synthetic organic chemistry.5,6 Both, heat and mass transfer is superior compared to traditional techniques and multiphasic reactions (e.g. gas/liquid,7 gas/liquid/solid,8 liquid/liquid9) can therefore be often dramatically improved. In addition, combustion and explosion hazards are reduced and, consequently, reactions in the explosive or thermal runaway regime can be exploited in a safe and controllable manner.
Carbon dioxide was recently intensively studied in its supercritical form (scCO2) as reaction medium during continuous processes.10 The use as gaseous reagent is comparably less studied, albeit several case studies show its potential in continuous manufacturing. One of the first examples was carried out using immobilized decarboxylase for the synthesis of pyrrole-2-carboxylates.11 The carboxylation of Grignard reagents using the tube-in-tube methodology has been studied with different continuous flow setups.12,13 Very recently, a lithium–halogen exchange reaction was combined with a subsequent carboxylation using the tube-in-tube gas addition concept in the continuous synthesis of amitryptiline.3 Leitner and coworkers were able to show that scCO2 can be continuously hydrogenated to pure formic acid using an immobilized catalyst.14 A novel synthesis of cyclic carbonates from epoxides and CO2 was recently presented by the Jamison laboratories.15 Interestingly, the only lithiation–carboxylation sequence of heterocycles reported so far was carried out for the conversion of furan and 2-chlorothiophene with n-BuLi and carbon dioxide at −30 °C (in case of the latter at 0 °C) resulting in the corresponding acids after 2–3.5 minutes.16
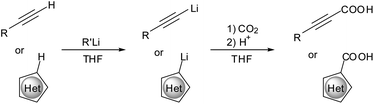
The present study was designed in order to develop a facile and efficient flash synthesis of carboxylic acids from terminal alkynes and heterocycles (Fig. 1) in a multistep continuous process. Therefore, the substrates were initially lithiated using suitable metalation agents. The organometallic intermediate was then treated with a well-defined amount of CO2 and subsequently quenched yielding the desired target molecules within a few seconds.
Phenylacetylene (1a) was chosen as model substrate to establish a setup for the metalation–carboxylation sequence with lithium bis(trimethylsilyl)amide (LiHMDS) and CO2 in a gas/liquid continuous flow regime. We initially optimized the parameters required for the carboxylation step by mixing a solution of LiHMDS and 1a in THF with the gaseous reagent in a T-mixing unit.17 After an intensive evaluation of various reaction parameters, including gas and liquid flow rates, residence time, temperature as well as back pressure regulation it was established that the carboxylate is formed in sufficient amounts within ∼0.5 seconds at room temperature. The next logical step was to combine the gas/liquid carbon dioxide fixation with an on-demand continuous flow generation of the reactive intermediate.17 Importantly, this could be easily implemented using an additional mixer in combination with a 0.1 mL residence time, resulting in the 4-feed approach shown in Fig. 2.18
In the final setup, a liquid stream of a commercially available 1 M solution of LiHMDS in THF was mixed with the alkyne dissolved in THF via a T-mixer. The flow rates and substrate concentration were set in order to obtain a slight excess of the lithium amide (1.1 equiv.). After a residence time of ∼3 seconds the lithium alkynate was mixed with the gaseous reagent in a second T-mixer resulting in a completely homogenous gas/liquid mixture at a back pressure of 10 bar. It has to be pointed out that the CO2 flow rate of 19 mLN min−1 corresponds to a comparably low excess (1.2 equiv.) and therefore constitutes an almost quantitative consumption of the greenhouse gas. However, we additionally realized that the performance of the CO2 mass flow controller had to be stabilized by preheating the gas to 65 °C in a stainless steel coil.19 High conversions of the organolithium intermediate to the corresponding carboxylate could be achieved within a short residence time of only ∼0.5 seconds. It is worth noting, that the residence time can be accurately controlled using a water quench before depressurization and collection of the reaction mixture. As small amounts of precipitates were formed either during the carboxylation, or after adding H2O a 1 mL coil was installed directly after the quench. During the time in which the reaction mixture passes this residence unit all solids dissolved, allowing the reaction mixture to smoothly pass the pressure-regulating unit.
With the optimized conditions in hand, we next evaluated our setup using several terminal alkynes (Table 1). It has to be noted that in all examples, small amounts of the substrate were still present in the reaction mixture as analyzed by HPLC-UV.20 Furthermore, we realized that 1–10% of a byproduct was formed during the reaction which could be identified as a trimethylsilane derivative apparently from a reaction of the base and the alkyne moiety. Electron-rich (1a, 1b) and electron poor (1c, 1d, 1f) aromatic alkynes were transformed into their corresponding carboxylic acids resulting in good yields after extraction of the byproducts followed by acidification and crystallization. Unfortunately, it was not possible to convert the phenol derivative 1e and 3-ethynylpyridine (1h) due to precipitation of either the organometallic intermediate or, in case of 1h, the carboxylate, resulting in a clogged reactor. However, we could expand the synthetic scope of our methodology by heterocyclic (1g) and aliphatic (1i, 1j) substrates without further modifications.
| Substrate | Conversionb (selectivity) (%) | Yieldc (%) | Substrate | Conversionb (selectivity) (%) | Yieldc (%) |
|---|---|---|---|---|---|
| a Reactions were carried out using 1.42 mmol alkyne, for general conditions see Fig. 2.b Determined as HPLC-UV peak area percent at 215 nm.c Isolated yield.d Not determined. | |||||
 |
93 (99) | 85 |  |
93 (98) | 89 |
 |
94 (92) | 81 |  |
91 (89) | 81 |
 |
91 (98) | 78 |  |
Clogging | — |
 |
97 (95) | 84 |  |
n.d.(n.d.)d | 90 |
 |
Clogging | — |  |
n.d.(n.d.) | 66 |
The propiolic acids obtained are valuable synthetic intermediates for a range of pharmaceutically or industrially relevant molecules, including polymers, coumarins, flavones, spirobenzofuranes, spiroindoles or vinyl sulfides.21
In order to broaden the synthetic horizon of the lithiation–carboxylation flow reactor concept, we decided to test the same methodology for its suitability to convert heterocycles into their corresponding carboxylic acids. Initially we decided to use the lithiation of thiophene (3a) by lithium diisopropylamide (LDA) with subsequent carboxylation for a feasibility study. In an initial experiment the reactor clogged immediately after the water quench which prompted us to change to a mixture of water and acetic acid (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to overcome this hurdle. Gratifyingly, thiophene-2-carboxylic acid (4a) could be isolated by extraction after this minor modification in satisfying yields without any further re-optimization of the continuous method (Fig. 3). An additional experiment using significantly higher amounts of CO2 (1.9 equiv.) resulted in only slightly higher amounts of the desired target molecule. Methyl substituted thiophenes (3b, 3c) as well as benzofuran (3f) provided moderate yields. Further optimization studies using 3b and either a higher excess of LDA (2 M, 2.2 equiv.) or larger amounts of carbon dioxide (30 mLN min−1, 1.9 equiv.) did not result in a significant improvement. In case of 1-phenylpyrazole the reactor immediately clogged after mixing with the organometallic base as the intermediate is apparently completely insoluble in THF. However, the use of electron poor thiophenes (4d, 4e) expanded the synthetic potential of the presented continuous flow methodology.
1) to overcome this hurdle. Gratifyingly, thiophene-2-carboxylic acid (4a) could be isolated by extraction after this minor modification in satisfying yields without any further re-optimization of the continuous method (Fig. 3). An additional experiment using significantly higher amounts of CO2 (1.9 equiv.) resulted in only slightly higher amounts of the desired target molecule. Methyl substituted thiophenes (3b, 3c) as well as benzofuran (3f) provided moderate yields. Further optimization studies using 3b and either a higher excess of LDA (2 M, 2.2 equiv.) or larger amounts of carbon dioxide (30 mLN min−1, 1.9 equiv.) did not result in a significant improvement. In case of 1-phenylpyrazole the reactor immediately clogged after mixing with the organometallic base as the intermediate is apparently completely insoluble in THF. However, the use of electron poor thiophenes (4d, 4e) expanded the synthetic potential of the presented continuous flow methodology.
In summary, we have developed a fast and efficient process for the continuous lithiation and subsequent carboxylation with carbon dioxide of terminal alkynes and heterocycles. The presented method provides valuable carboxylic acids within ∼3.5 seconds reaction time using low excess of the organometallic base and the gaseous reagent in moderate to good isolated yields.
Acknowledgements
This work was supported by a grant from the Christian Doppler Research Society (CDG). The authors gratefully thank ThalesNano Nanotechnology Inc. for providing the H-Cube Gas Module™ and the technical support.Notes and references
- (a) T. Sakaura, J.-C. Choi and H. Yasuda, Chem. Rev., 2007, 107, 2365 CrossRef PubMed; (b) M. Aresta and A. Dibeneedetto, Dalton Trans., 2007, 2975 RSC; (c) I. Omae, Coord. Chem. Rev., 2012, 256, 1384 CrossRef CAS PubMed; (d) X. Cai and B. Xie, Synthesis, 2013, 45, 3305 CrossRef CAS PubMed.
- (a) A. Nagaki, D. Ichinari and J. Yoshida, Chem. Commun., 2013, 49, 3242 RSC; (b) A. Nagaki, Y. Uesugi, H. Kim and J. Yoshida, Chem.–Asian J., 2013, 8, 705 CrossRef CAS PubMed; (c) A. Nagaki, Y. Takahashi, S. Yamada, C. Matsuo, S. Haraki, Y. Moriwaki, S. Kim and J. Yoshida, J. Flow Chem., 2012, 2, 70 CrossRef CAS; (d) A. Nagaki, Y. Moriwaki, S. Haraki, A. Kenmoku, A. Hayashi and J. Yoshida, Chem.–Asian J., 2012, 7, 1061 CrossRef CAS PubMed.
- L. Kupracz and A. Kirschning, Adv. Synth. Catal., 2013, 355, 3375 CrossRef CAS.
- (a) J. Yoshida, Y. Takahashi and A. Nagaki, Chem. Commun., 2013, 49, 9896 RSC; (b) J. Yoshida, Chem. Rec., 2010, 10, 323 Search PubMed; (c) J. Yoshida, Flash Chemistry: Fast Organic Synthesis in Microsystems, Wiley-Blackwell, Oxford, U.K., 2008 Search PubMed.
- For selected books see: (a) W. Ehrfeld, V. Hessel and H. Lowe, Microreactors, Wiley-VCH, Weinheim, Germany, 2000 CrossRef; (b) V. Hessel, S. Hardt and H. Lowe, Chemical Micro Process Engineering, Wiley-VCH, Weinheim, Germany, 2004 Search PubMed; (c) V. Hessel, A. Renken, J. C. Schouten and J. Yoshida, Micro Process Engineering, Wiley-Blackwell, Oxford, U.K., 2009 Search PubMed; (d) T. Wirth, Microreactors in Organic Synthesis and Catalysis, Wiley-VCH, Weinheim, Germany, 2nd edn, 2013 Search PubMed.
- For recent reviews see: (a) R. L. Hartman, J. P. McMullen and K. F. Jensen, Angew. Chem., Int. Ed., 2011, 50, 7502 CrossRef CAS PubMed; (b) J. Wegner, S. Ceylan and A. Kirschning, Adv. Synth. Catal., 2012, 354, 17 CrossRef CAS; (c) C. Wiles and P. Watts, Green Chem., 2012, 14, 38 RSC; (d) S. G. Newman and K. F. Jensen, Green Chem., 2013, 15, 1456 RSC; (e) S. C. Stouten, T. Noel, Q. Wang and V. Hessel, Aust. J. Chem., 2013, 66, 121 CrossRef CAS.
- For recent publications on continuous gas/liquid chemistry from our laboratories, see: (a) B. Gutmann, P. Elsner, D. Roberge and C. O. Kappe, ACS Catal., 2013, 3, 2669 CrossRef CAS; (b) F. Mastronardi, B. Gutmann and C. O. Kappe, Org. Lett., 2013, 16, 5590 CrossRef PubMed; (c) B. Pieber, S. T. Martinez, D. Cantillo and C. O. Kappe, Angew. Chem., Int. Ed., 2013, 52, 10241 CrossRef CAS PubMed; (d) B. Pieber and C. O. Kappe, Green Chem., 2013, 15, 320 RSC.
- For a recent publications on continuous gas/liquid/solid chemistry from our laboratories, see: D. Obermayer, A. M. Balu, A. A. Romero, W. Goessler, R. Luque and C. O. Kappe, Green Chem., 2013, 15, 1530 RSC.
- For recent publications on continuous liquid/liquid chemistry from our laboratories, see: (a) B. Reichart, T. N. Glasnov and C. O. Kappe, Synlett, 2013, 24, 239 Search PubMed; (b) M. Damm, B. Gutmann and C. O. Kappe, ChemSusChem, 2013, 6, 978 CrossRef CAS PubMed.
- (a) U. Hintermair, G. Francio and W. Leitner, Chem.–Eur. J., 2013, 19, 4538 CrossRef CAS PubMed; (b) J. Theuerkauf, G. Francio and W. Leitner, Adv. Synth. Catal., 2013, 355, 209 CrossRef CAS; (c) M. J. Casciato, G. Vevitin, D. W. Hess and M. A. Grover, ChemSusChem, 2012, 5, 1186 CrossRef CAS PubMed; (d) M. Selva, S. Guidi, A. Perosa, M. Signoretto, P. License and T. Maschmeyer, Green Chem., 2012, 14, 2727 RSC.
- T. Matsuda, R. Marukado, S. Koguchi, T. Nagasawa, M. Mukouyama, T. Harada and K. Nakamura, Tetrahedron Lett., 2008, 49, 6019 CrossRef CAS PubMed.
- A. Polyzos, M. O'Brien, T. P. Petersen, I. R. Baxendale and S. V. Ley, Angew. Chem., Int. Ed., 2011, 50, 1190 CrossRef CAS PubMed.
- J. J. F. van Gool, S. A. M. W. van den Broek, R. M. Ripken, P. J. Nieuwland, K. Koch and F. P. J. T. Rutjes, Chem. Eng. Technol., 2013, 36, 1042 CrossRef CAS.
- S. Wesselbaum, U. Hintermair and W. Leitner, Angew. Chem., Int. Ed., 2012, 51, 8585 CrossRef CAS PubMed.
- J. A. Kozak, J. Wu, F. Simeon, T. A. Hatton and T. F. Jamison, J. Am. Chem. Soc., 2013, 153, 18497 CrossRef PubMed.
- S. Buchholz, C. Severins, K. Tellmann, K. Weidemann and J. Wieschemeyer, Ger. Pat., DE102009060033, 2011.
- A detailed summary of the optimization see Table S1& S2 in the ESI.†.
- For a detailed description including images of the continuous flow set up, see ESI.†.
- The mass flow controller was not able to control the gaseous reagent in a stable fashion, presumably due to condensation.
- A representative HPLC chromatogram is given in the ESI (Fig. S3).†.
- F. Manjolinho, M. Arndt, K. Gooßen and L. J. Gooßen, ACS Catal., 2012, 2, 2014 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: General information, detailed description of the equipment, optimization data, synthetic procedures, product characterization, copies of NMR spectra. See DOI: 10.1039/c4ra01442a |
| This journal is © The Royal Society of Chemistry 2014 |