The role of substrate interactions in the modification of surface forces by self-assembled monolayers
Abstract
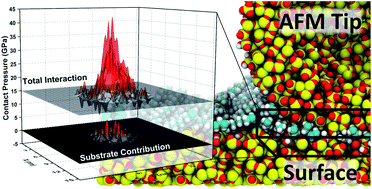
Self-assembled monolayers have been used extensively as surface modifications and model systems for friction and adhesion mitigation on surfaces. From experiment, it is unclear to what extent and under what conditions the substrate plays a role in the modification of these surface forces, but because SAMs are relatively compliant and thin, it is reasonable to assume that the unique frictional characteristics of these monolayers is driven in part by substrate effects. Molecular dynamics simulation and methods developed for analysis of total interaction area, and direct substrate interaction, have been employed to investigate the structure of surface asperity contacts coated with SAMs, examining these interactions and determining what role substrate interactions and other possible dissipation mechanisms are involved in the friction response of SAMs. It was observed that for sparse OTS films, typical of films formed on rough or asperity surfaces, substrate interactions are extensive, leading to increased tribochemistry and strain at sliding interfaces. For densely packed films, it was found that even pressures on the order of a few GPa do not lead to direct substrate interaction, but there is a distinct and localized increase in the compressive strain on the film, indicating the development of new dissipative modes during sliding at high pressures including conformational changes and wear of the films.
- This article is part of the themed collection: Tribology

 Please wait while we load your content...
Please wait while we load your content...