A new route to dithia- and thiaoxacyclooctynes via Nicholas reaction†
Tobias Hagendorna and
Stefan Bräse*ab
aInstitute of Organic Chemistry, Karlsruhe Institute of Technology, Fritz-Haber-Weg 6, 76131 Karlsruhe, Germany. E-mail: stefan.braese@kit.edu
bInstitute of Toxicology and Genetics, KIT, Campus North, Hermann-von-Helmholtz-Platz 1, 76344 Eggenstein-Leopoldshafen, Germany
First published on 19th March 2014
Abstract
The current paper describes a new synthesis of heteroatom-substituted cyclooctynes. By using the Nicholas reaction we managed to design a concise synthesis that only uses three steps to build the eight-membered ring. It was also possible to functionalize said alkyne with a fluorophore.
Cyclooctynes have developed into an important class of compounds that are used as highly versatile tools in chemical biology.1–13 They are applied as chemical reporters in strain-promoted click reactions to label various kinds of biomolecules like glycans,14 proteins15 and DNA16,17 as well as in materials sciences.18 Their synthesis usually comprises many steps, the final one being the generation of the triple bond. Widespread reactions that are used to obtain the alkyne moiety are elimination reactions starting from vinyl bromides,19 ketones,20 enol triflates2 and related elimination reaction precursors. Furthermore there are other possibilities like the oxidation of dihydrazones,21 thermal decomposition of selenadiazols22 or the irradiation of cyclopropenones.23 The incorporation of an intact alkyne group through a ring closing reaction is hardly ever used because the ring strain building up during the course of reaction usually prevents the ring formation. There are examples, however, where heteroatom-substituted cyclooctynes were synthesized by SN2 ring formation between 1,2-ethanedithiol and 1,4-dibromo-2-butyne as shown by Meier et al.24 This type of reaction, though possible, results in considerable by-product formation. Several reactions were reported which use the Nicholas reaction to build up similar (macro-)cyclic compounds.25–27 The Nicholas reaction is a reaction starting from a dicobalt hexacarbonyl–alkyne complex bearing an oxygen atom in the propargylic position.28–30 By adding an acid it is very easy to generate a carbocation in the propargylic position that can then in turn react with a suitable nucleophile. To our knowledge the scope of the double Nicholas reaction to generate (hetero)cyclooctynes in one step has not been fully explored.31–41 However, as mentioned above, there have been syntheses of cyclooctyne dicobalt complexes that were used directly in various transformations, i.e. Pauson–Khand reactions, without generating the decomplexed alkyne.42–44
The route started with the synthesis of the cobalt complexes of 1,4-dithia-6-cyclooctyne and 1-thia-4-oxa-6-cyclooctyne using the starting material 1,4-but-2-ynediol as described by Went et al.27 Therefore starting complex 1 (ref. 45) was dissolved in dichloromethane, the nucleophile (2 or 3 respectively) and finally HBF4-etherate as the acid catalyst were added and the mixture was stirred at r.t. for 48 h. The resulting complexes were then treated directly with ferric nitrate in methanol and leading to the cyclooctynes 4 and 5 as oils exhibiting a typical odor. Alkynes 4 and 5 were obtained in 26% and 13% yield respectively (Scheme 1). Comparable yields were obtained for the formation of a less strained cyclononyne by Young et al.36
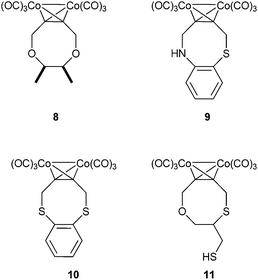
The dithiacyclooctyne 4 was then treated with benzyl azide to accomplish a strain promoted click-reaction. In our attempts to react the thia-oxa cyclooctyne we chose tetracyclone as a reactant to avoid the formation of regioisomers. In both cases the reactions worked out, yielding the expected products. We then tried to vary the scope of the reaction by changing the nucleophile to a glycol derivative, bearing only oxygen atoms and no sulphur. Glycol itself proved to be an unsuitable reaction partner in the Nicholas reaction, as did pinacol, glycerol and methyl glycerate. It was possible, however, to observe a reaction with meso-1,2-dimethylglycol, affording the corresponding dicobalt hexacarbonyl complex (8). However, the deprotection to the free alkyne did not yield the anticipated cyclooctyne.
Besides the aliphatic dithiols, there are also aromatic dithiols that were used in literature procedures46,47 to build up macrocyclic dicobalt–alkyne complexes. We therefore also investigated the use of 1,2-benzenedithiols, o-mercaptophenol, 2-aminothiophenol and 3,4-dihydroxy methyl benzoate. We observed no product formation for the reaction of the aromatic diols. And also o-mercaptophenol could not be reacted successfully to the desired product. We were not able to identify the side products, that were formed but we assume that the generated cations react in an electrophilic aromatic substitution. However, it was possible to isolate the products of the reaction between the dicobalt hexacarbonyl complex of 1,4-but-2-ynediol (1) and 1,2-benzenedithiol or 2-aminothiophenol, the complexes 10 and 9, respectively, both in acceptable yields (51% and 31%).
Some of the complexes are not suitable for mild deprotection. The standard procedure for the oxidation of the complexes to the free alkyne used 5.00 equivalents of ferric nitrate in a methanolic solution at room temperature. Under the conditions the free cyclooctyne could not be observed in cases of complex 9 and 10. Also the variation of reaction conditions (e.g. lower temperature and different amounts of the oxidizing agent) did not yield the desired metal-free cyclooctynes. We then tried to use the known cyclooctyne scavenger tetracyclone to determine whether the alkyne was formed at all during the reaction. Interestingly we were able to detect the masses of both products in the crude product, showing that the deprotection indeed worked. However, even after the detection of the scavenged product in the MS-spectra neither the alkyne nor the alkyne–tetracyclone reaction products could be isolated from the respective reactions' raw products.
After the aromatic nucleophiles, we wanted to explore the possibilities to attach an additional functional group, in order to have a means to functionalize the alkyne. We studied further possible substances and used 2,3-dimercapto-1-propanol and 1-thioglycerol (12) to yield cyclooctynes with an additional alcohol group. The reaction with 2,3-dimercapto-1-propanol was investigated first, expecting that the product would be the dithiacyclooctyne-ring due to the higher nucleophilicity of the sulfur. The reaction worked but when we examined the NMR spectra we realized that the wrong isomer was formed. Instead of the desired dithia-product we were able to synthesize the thia-oxa-product (11, Fig. 1). Due to the fact that the free thiol is prone to oxidation under oxidizing deprotection conditions, it was not suitable for further functionalization. Therefore the alternative 1-thioglycerol (12) was used and thus the desired complex 13 could be obtained and be further deprotected to the cyclooctyne 14 (Scheme 2). To further functionalize it, we used carbonyldiimidazole to attach fluorescein-piperazine-amide as a carbamate. Thus we were able to synthesize a new cyclooctyne dye conjugate 15 in only four steps. Such cyclooctyne–dye conjugates are useful tools in cell labelling.
In summary, we were able to devise a new route to heterocyclooctynes via Nicholas reaction. Thus we were able to synthesize heteroatom-cyclooctynes in only three steps and a dye-functionalized cyclooctyne in four steps, starting from commercially available 1,4-dihydroxy-but-2-yne. This sequence is one of the shortest syntheses of the cyclooctyne scaffold to date.
We would like to thank the Landesgraduiertenförderung Baden–Württemberg for financial support and Danny Wagner (KIT) for dedicated experimental help.
Notes and references
- J. M. Baskin, J. A. Prescher, S. T. Laughlin, N. J. Agard, P. V. Chang, I. A. Miller, A. Lo, J. A. Codelli and C. R. Bertozzi, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 16793–16797 CrossRef CAS PubMed.
- J. A. Codelli, J. M. Baskin, N. J. Agard and C. R. Bertozzi, J. Am. Chem. Soc., 2008, 130, 11486–11493 CrossRef CAS PubMed.
- P. Kele, X. Li, M. Link, K. Nagy, A. Herner, K. Lorincz, S. Beni and O. S. Wolfbeis, Org. Biomol. Chem., 2009, 7, 3486–3490 CAS.
- P. V. Chang, J. A. Prescher, E. M. Sletten, J. M. Baskin, I. A. Miller, N. J. Agard, A. Lo and C. R. Bertozzi, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 1821–1826 CrossRef CAS PubMed.
- E. M. Sletten, H. Nakamura, J. C. Jewett and C. R. Bertozzi, J. Am. Chem. Soc., 2010, 132, 11799–11805 CrossRef CAS PubMed.
- R. D. Carpenter, S. H. Hausner and J. L. Sutcliffe, ACS Med. Chem. Lett., 2011, 2, 885–889 CrossRef CAS PubMed.
- J. C. Jewett and C. R. Bertozzi, Org. Lett., 2011, 13, 5937–5939 CrossRef CAS PubMed.
- B. C. Sanders, F. Friscourt, P. A. Ledin, N. E. Mbua, S. Arumugam, J. Guo, T. J. Boltje, V. V. Popik and G.-J. Boons, J. Am. Chem. Soc., 2011, 133, 949–957 CrossRef CAS PubMed.
- E. M. Sletten and C. R. Bertozzi, Acc. Chem. Res., 2011, 44, 666–676 CrossRef CAS PubMed.
- H. E. Bostic, M. D. Smith, A. A. Poloukhtine, V. V. Popik and M. D. Best, Chem. Commun., 2012, 48, 1431–1433 RSC.
- C. G. Gordon, J. L. Mackey, J. C. Jewett, E. M. Sletten, K. N. Houk and C. R. Bertozzi, J. Am. Chem. Soc., 2012, 134, 9199–9208 CrossRef CAS PubMed.
- M. R. Karver, R. Weissleder and S. A. Hilderbrand, Angew. Chem., Int. Ed., 2012, 51, 920–922 CrossRef CAS PubMed , S920/921-S920/912.
- A. A. Neves, H. Stockmann, Y. A. Wainman, J. C. H. Kuo, S. Fawcett, F. J. Leeper and K. M. Brindle, Bioconjugate Chem., 2013, 24, 934–941 CrossRef CAS PubMed.
- N. J. Agard, J. A. Prescher and C. R. Bertozzi, J. Am. Chem. Soc., 2004, 126, 15046–15047 CrossRef CAS PubMed.
- M. F. Debets, S. S. van Berkel, S. Schoffelen, F. P. J. T. Rutjes, J. C. M. van Hest and F. L. van Delft, Chem. Commun., 2010, 46, 97–99 RSC.
- I. Singh and F. Heaney, Chem. Commun., 2011, 47, 2706–2708 RSC.
- I. Singh, J. S. Vyle and F. Heaney, Chem. Commun., 2009, 3276–3278 RSC.
- Z. B. Wang, J. X. Liu, H. K. Arslan, S. Grosjean, T. Hagendorn, H. Gliemann, S. Bräse and C. Wöll, Langmuir, 2013, 29, 15958–15964 CrossRef CAS PubMed.
- L. Brandsma and H. D. Verkruijsse, Synthesis, 1978, 290 CrossRef CAS.
- M. K. Schultz, S. G. Parameswarappa and F. C. Pigge, Org. Lett., 2010, 12, 2398–2401 CrossRef CAS PubMed.
- G. Wittig and A. Krebs, Chem. Ber., 1961, 94, 3260–3275 CrossRef CAS.
- H. Meier and H. Gugel, Synthesis, 1976, 338–339 CrossRef CAS.
- A. A. Poloukhtine, N. E. Mbua, M. A. Wolfert, G.-J. Boons and V. V. Popik, J. Am. Chem. Soc., 2009, 131, 15769–15776 CrossRef CAS PubMed.
- H. Meier and Y. Dai, Tetrahedron Lett., 1993, 34, 5277–5280 CrossRef CAS.
- A. Gelling, J. C. Jeffery, D. C. Povey and M. J. Went, Chem. Commun., 1991, 349–351 RSC.
- G. F. Mohmand, K. Thiele and M. J. Went, J. Organomet. Chem., 1994, 471, 241–248 CrossRef CAS.
- A. Gelling, G. F. Mohmand, J. C. Jeffery and M. J. Went, Dalton Trans., 1993, 1857–1862 RSC.
- R. F. Lockwood and K. M. Nicholas, Tetrahedron Lett., 1977, 18, 4163–4165 CrossRef.
- K. M. Nicholas and R. Pettit, Tetrahedron Lett., 1971, 12, 3475–3478 CrossRef.
- J. R. Green, Curr. Org. Chem., 2001, 5, 809–826 CrossRef CAS.
- M. A. Bennett and P. B. Donaldson, Inorg. Chem., 1978, 17, 1995–2000 CrossRef CAS.
- T. F. Jamison, S. Shambayati, W. E. Crowe and S. L. Schreiber, J. Am. Chem. Soc., 1994, 116, 5505–5506 CrossRef CAS.
- L. J. Hope-Weeks, M. J. Mays and G. A. Solan, Eur. J. Inorg. Chem., 2007, 3101–3114 CrossRef CAS.
- R. C. J. Atkinson, L. J. Hope-Weeks, M. J. Mays and G. A. Solan, J. Organomet. Chem., 2007, 692, 2076–2085 CrossRef CAS.
- Y. F. Lu and J. R. Green, Synlett, 2001, 243–247 CAS.
- D. G. J. Young, J. A. Burlison and U. Peters, J. Org. Chem., 2003, 68, 3494–3497 CrossRef CAS PubMed.
- K. Mitachi, T. Shimizu, M. Miyashita and K. Tanino, Tetrahedron Lett., 2010, 51, 3983–3986 CrossRef CAS.
- N. Iwasawa, K. Inaba, S. Nakayama and M. Aoki, Angew. Chem., Int. Ed., 2005, 44, 7447–7450 CrossRef CAS PubMed.
- S. Nagumo, Y. Ishii, G. Sato, M. Mizukami, M. Imai, N. Kawahara and H. Akita, Tetrahedron Lett., 2009, 50, 26–28 CrossRef CAS.
- A. Nonoyama, A. Hamajima and M. Isobe, Tetrahedron, 2007, 63, 5886–5894 CrossRef CAS.
- N. Itoh, T. Iwata, H. Sugihara, F. Inagaki and C. Mukai, Chem.–Eur. J., 2013, 19, 8665–8672 CrossRef CAS PubMed.
- C. Mukai, T. Kojima, T. Kawamura and F. Inagaki, Tetrahedron, 2013, 69, 7659–7669 CrossRef CAS.
- K. D. Closser, M. M. Quintal and K. M. Shea, J. Org. Chem., 2009, 74, 3680–3688 CrossRef CAS PubMed.
- S. Djurdjevic and J. R. Green, Org. Lett., 2013, 15, 5468–5471 CrossRef CAS PubMed.
- M. Gruselle, B. Malézieux, J. Vaissermann and H. Amouri, Organometallics, 1998, 17, 2337–2343 CrossRef CAS.
- L. J. Hope-Weeks, M. J. Mays and A. D. Woods, Dalton Trans., 2002, 1812–1819 RSC.
- V. B. Golovko, M. J. Mays and G. A. Solan, J. Organomet. Chem., 2007, 692, 4985–4994 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra01345j |
| This journal is © The Royal Society of Chemistry 2014 |