Improvement of electrochemical performance of rechargeable lithium–selenium batteries by inserting a free-standing carbon interlayer†
Abstract
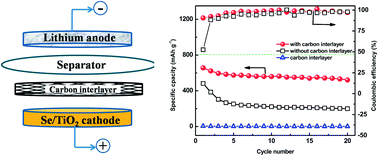
A simple, low-cost modification of lithium–selenium (Li–Se) cells by placing a carbon interlayer between the selenium electrode and the separator has been investigated to significantly improve the electrochemical performance of Li–Se cells.

 Please wait while we load your content...
Please wait while we load your content...