Carbon-coated hierarchically porous silicon as anode material for lithium ion batteries
Lanyao Shen,
Zhaoxiang Wang* and
Liquan Chen
Key Laboratory for Renewable Energy, Chinese Academy of Sciences, Beijing Key Laboratory for New Energy Materials and Devices, Beijing National Laboratory for Condensed Matter Physics, Institute of Physics, Chinese Academy of Sciences, Beijing, 100190, China. E-mail: zxwang@iphy.ac.cn
First published on 12th March 2014
Abstract
Silicon is one of the most promising anode materials for high energy density lithium ion batteries. However, its fast capacity decay due to volume expansion and the resultant particle pulverization hinders its commercial application. Diverse strategies have been proposed to reduce these effects. In this article, hierarchically porous silicon composite is formed by coating the porous silicon particles obtained by magnesiothermically reducing commercial diatomite. Both the void space between the silicon particles and the pores within each silicon particle are designed to accommodate the volume change of silicon in the charge and discharge processes. On the other hand, the carbon coating layer alleviates the volume change of silicon and maintain the electric contact between the particles. The carbon-coated hierarchically porous silicon composite based on this design concept shows much better cyclability than that of the bare porous silicon.
Introduction
Silicon is one of the most promising anode materials to replace the commercially used graphite anode materials of lithium ion batteries due to its large theoretical capacity of 4200 mA h g−1.1 However, commercialization of silicon as an anode material has been significantly hindered due to severe volume expansion and fast capacity fading. Both of these two factors are closely related to the volume variation of silicon and, therefore, its particle pulverization, during lithium insertion and extraction. As a result, suppression of the particle pulverization becomes the key to the improvement of the performances of the silicon anode and the silicon-anode based lithium ion batteries.Comprehensive studies have been carried out to construct special nanostructures,2,3 nanowires,4,5 nanotubes,6,7 thin films8 and porous silicon with conductive coating layer to suppress the particle pulverization.9–13 Esmanski et al.14 proposed to use inverse-opal-based macroporous silicon as an anode material. It delivers capacity retentions over 80% after 145 cycles. Yao et al.15 reported interconnected silicon hollow spheres with long cycle life. However, it might be difficult for these methods to find a real application due to both the complicated fabrication process and the expensive starting materials.
Recently, numerous micro-sized Si–C composites have been prepared with low-cost and scalable approaches as promising anode materials for lithium ion batteries. Park et al.16,17 made a micro-sized porous Si–C composite by catalytically etching the surface layers of micro-sized Si followed by carbon coating. The obtained micro-sized porous Si–C powder shows good cycling stability for 70 cycles. Yi et al.18 prepared a micro-sized Si–C composite by heating SiO, etching the resultant SiO2 and carbon-coating the residual Si. In our previous report,19 porous silicon was obtained by magnesiothermically reducing commercial diatomite. Such prepared silicon was proved to be a high-capacity anode material for lithium ion batteries. However, fast capacity decay still occurs during cycling due to insufficient room that the mesopores could provide for volume expansion and the poor electric contact between the pulverized particles. In this work, carbon-coated silicon composite with hierarchically porous structure was prepared. With the void space between the silicon particles and the mesopores within each silicon particle, this carbon-coated silicon composite supplies sufficient room to accommodate the volume change of silicon and, therefore, shows much improved cycling stability.
Experimental
Preparation of porous silicon and silicon–carbon composite
Porous silicon was obtained by magnesiothermically reducing commercial diatomite as described in our previous report,19 except that the mixture of diatomite and magnesium (Mg) powders was ball-milled at a speed of 350 rpm for 5 h to decrease the particle size of the diatomite and to get a homogeneous mixture before the magnesiothermic reaction at 650 °C for 6 h under Ar/H2 mixed atmosphere. In a typical preparation, 1.0 g of the porous silicon, 0.05 g of carbon black, 0.3 g of phenolic resin, and 50 ml of ethanol were magnetically stirred and ultrasonically dispersed to get a homogeneous suspension. The suspension was then sprayed in a vertical type spray drying machine with air flow rate of 10 ml min−1. The inlet and outlet temperatures were 80 °C and 68 °C, respectively. The obtained precursor was annealed in a tube furnace at 800 °C for 5 h under Ar atmosphere at a heating rate of 5 °C min−1. After the tube furnace was cooled down to room temperature, the carbon-coated hierarchically porous silicon composite was obtained.Characterization and electrochemical measurement
X'Pert Pro MPD X-ray diffractometer and Renishaw inVia micro-Raman spectroscopy was employed for structural characterization. The specific surface area of the sample was studied by the N2 absorption Brunauer–Emmett–Teller method. The carbon content of the composite was determined by inductively coupled plasma (ICP) analysis. SEM (Hitachi S-4800) and TEM (Tecnai G2 F20 U-TWIN) was used for morphology observation. The working electrode consists of 60 wt% active material (the silicon/carbon composite), 20 wt% carbon black and 20 wt% sodium alginate. First, proportional active material, carbon black and sodium alginate were mixed in water to form homogenous slurry. The slurry was then spread on a Cu foil by a doctor's blade method. After the working electrode was dried in a vacuum oven at 100 °C for 6 h to remove the trace of water, coin-type test cells were assembled in an Ar-filled glove box (both the O2 and H2O contents are lower than 0.1 ppm) with Celegard 2400 as the separator and 1 mole L−1 LiPF6 in EC/DMC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) as the electrolyte. Lithium foil was used as the counter electrode. Galvanostatic cycling and rate performance were performed on a Land battery tester (Wuhan, China) between 0.005 and 1.5 V at room temperature.
1 v/v) as the electrolyte. Lithium foil was used as the counter electrode. Galvanostatic cycling and rate performance were performed on a Land battery tester (Wuhan, China) between 0.005 and 1.5 V at room temperature.
Results and discussion
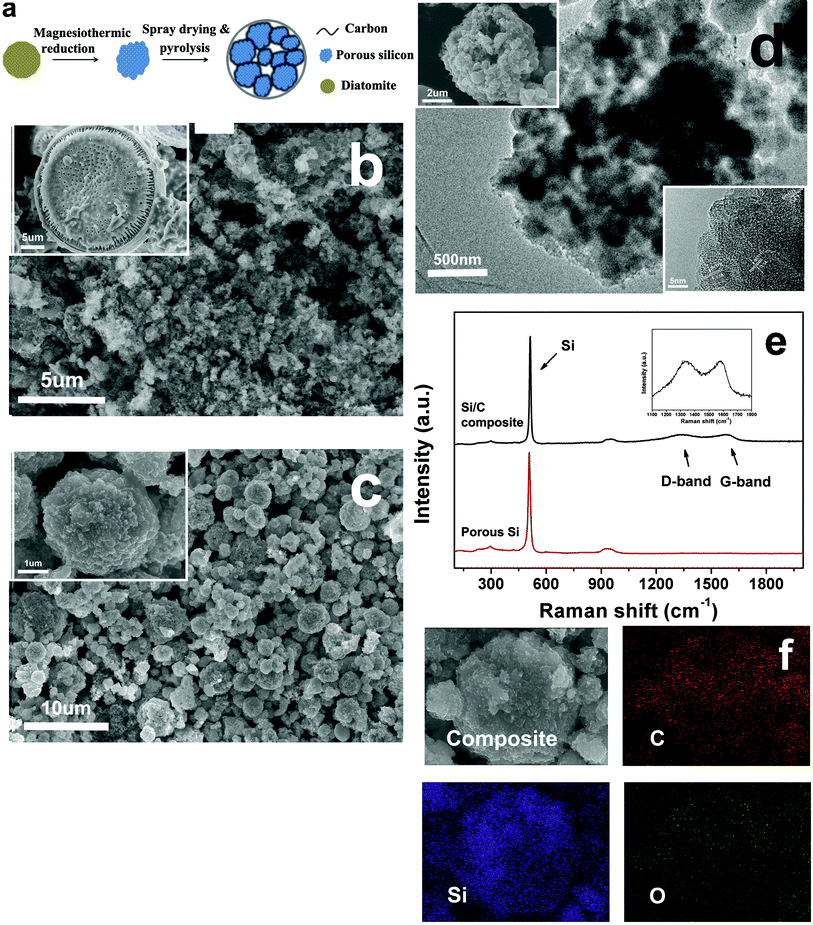
Fig. 1a shows the schematic diagram of the synthesis process of the carbon-coated hierarchically porous silicon composite. The as-received diatomite, like a sun-flower, is more than 20 μm in diameter (inset of Fig. 1b). In contrast, the size of the silicon particle after ball-milling and subsequent magnesiothermic reduction of the diatomite is less than 2 μm (Fig. 1b), beneficial for the improvement of the cycling performance. Fig. 1c shows the spherical structure of the carbon-coated hierarchically porous silicon composite.The porous structure of the silicon is confirmed with TEM imaging (Fig. 1d). The interplanar spacing (inset in Fig. 1d) of the crystallite was measured to be 3.1 Å, corresponding to that of the Si (111) facet. Based on the above results, both the void space between porous Si particles in the composite and mesopores within the Si particles are expected to effectively accommodate the large volume change during cycling.
Raman spectroscopy, sensitive to both crystalline and amorphous species, is applied to investigate the structure of the component in the composite. The strong peak around 518 cm−1 and all the other peaks below 1000 cm−1 in Fig. 1e can be attributed to elemental Si.20 This means that all the diatomite has been successfully reduced to Si by the magnesiothermic reaction. The broad peaks at 1340 cm−1 and 1589 cm−1 are for the D- and G-bands of carbons, respectively, indicating the amorphous feature of the carbon in the composite. The carbon content of the composite is determined to be about 15 wt% by ICP analysis.
Energy dispersive spectroscopy mapping was used to explore the distribution of the elements in the composite. The mapping images of the spherical Si–C composites (Fig. 1f) show a uniform distribution of the Si and C elements, implying the even coating of carbon on the silicon. Carbon coating enhances the conductivity of the composite and helps to maintain the electric contact between the porous Si particles. The residual oxygen of the pyrolyzed phenolic resin (C6H6O) and, probably, slight oxidation of the porous silicon are believed to be responsible for the detected oxygen.
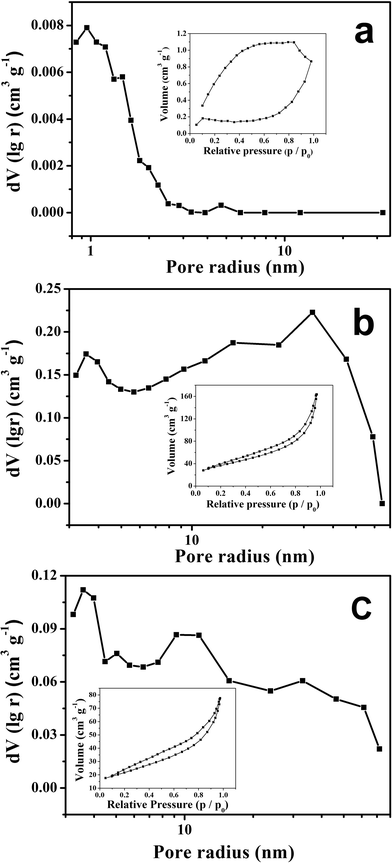
Fig. 2 compares the pore size distribution of the commercial diatomite, the as-prepared porous silicon and carbon coated porous silicon. It is found that the size of most of the pores increases from about 1 nm in the diatomite to 10–70 nm in the porous silicon. The significantly increased pore size in the porous silicon indicates that mesopores are created during the magnesiothermic reduction and subsequent removal of the by-products by acid immersion. Nitrogen adsorption analysis (inset of Fig. 2) indicates that the obtained porous Si delivers a specific surface area up to 131 m2 g−1, in comparison with that of the as-received diatomite, 1 m2 g−1. Both the small particle size and porous structure of the Si contribute to the drastic increase of the specific surface area, favourable for enhancing the kinetic properties of the electrode. SBA-15, usually applied as a template for preparing nano-scaled materials, was once reduced for porous silicon,10,13 due to its special porous structure and high surface area. However, the high cost hinders its practical application. Therefore, based on the above characterization, the pore-creating effect of the magnesiothermic reduction and subsequent acid immersion process, other low-cost silicon sources such as silica fume can also be used to prepare porous silicon for anode materials. Nitrogen adsorption analysis was also conducted to find out the influence of carbon coating on the surface physics of the porous silicon. Fig. 2c shows that the specific surface area of the carbon coated porous silicon is 76 m2 g−1, smaller than the bare porous silicon. Besides, the pore size distribution analysis indicates that the pore volume of macropores of the carbon coated porous silicon in the range of 20–60 nm is evidently reduced compared to the bare porous silicon, indicating the efficacy of the carbon coating layer.
Fig. 3a inset illustrates the potential profiles of the carbon-coated hierarchically porous Si composite between 0.005 and 1.5 V at a current density of 0.1 A g−1 (∼0.1 C). The potential profiles show characteristic lithiation and de-lithiation plateaus of crystalline silicon. The specific discharge and charge capacities in the first cycle are 2547 and 1716 mA h g−1, respectively. The irreversible capacity is attributed to the electrolyte decomposition and the trapping of some lithium ions in the defects of the carbon and, probably, some pulverized silicon fragments losing electric contact with others. Differential capacity curves (dQ/dV, Fig. 3a) of some selected cycles show some typical lithiation and delithiation peaks of amorphous Si. The 0.08 V and 0.21 V peaks are for the lithium insertion and 0.31 and 0.50 V peaks are for the lithium extraction.21 The similarity of the dQ/dV curves of different cycles implies the good capacity retention of the composite.
To investigate the impacts of the hierarchically porous structure of the composite on the electrochemical performance, the cycling capability of the as-prepared (bare) porous Si and carbon-coated hierarchically porous silicon composite is compared in Fig. 3b. The specific capacity of both electrodes is calculated based on the weight of the active material (silicon for the former, composite for the latter). The current density was 0.1 A g−1 for first cycle but 0.4 A g−1 for the subsequent cycles. Although the capacity of the bare porous silicon is higher than that of the carbon-coated hierarchically porous silicon composite in the first cycle due to the presence of low-capacity carbon in the latter, the capacity of the bare porous Si drops to only about 205 mA h g−1 after 110 cycles. In contrast, 920 mA h g−1 of the capacity is retained for the hierarchical composite due to the improved electric contact by its carbon conducting network. In addition, the coulombic efficiency of the hierarchical composite is also much higher than that of bare porous silicon in the first 20 cycles. It can be ascribed that the hierarchically porous structure and carbon coating layer of the composite can effectively suppress the pulverization of silicon, facilitating the formation of a stable solid electrolyte interphase (SEI) layer. As for the bare porous silicon, repeat particle cracks in the first few cycles lead to continuous fresh silicon surface exposing to the electrolyte, resulting in low coulombic efficiency. Moreover this hierarchically porous carbon-coated silicon shows much superior cycling performance over other Si–C composites using diatomite as the silicon source.12,19 This is ascribed to the binding and buffering effects of the carbon coating layer that maintains the electric contact of each particle and particles to the current collector and the hierarchically porous structure that accommodates the large volume change during cycling. These two effects enhance the integrity of the electrode and ensure the high capacity retention of the silicon electrode.
Fig. 3c shows the charge and discharge profiles and rate performance of the carbon-coated hierarchically porous silicon composite at different current densities. It delivers reversible capacities of about 1700, 1560, 1300, 1000 and 850 mA h g−1 at a current density of 0.1, 0.4, 0.8, 1.6 and 3.2 C, respectively, showing a good rate capability.
As diatomite is abundant and commercially available, magnesiothermic reduction is simple and inexpensive, and spray drying and subsequent carbonization can be easily industrialized, it is reasonable to believe that this carbon-coated hierarchically porous silicon composite can be an attractive anode material for lithium ion batteries.
Conclusions
Carbon-coated hierarchically porous silicon was prepared using an industrial method. The carbon coating layer, void space between the Si particles and the mesopores within the Si particles contribute to the improved cycling capability and rate performance of the silicon. By optimizing the binder or decreasing the particle size of the silica, the rate performance and capacity retention of the carbon-coated hierarchically porous silicon can be further improved, leading to a promising anode material for lithium ion batteries.Acknowledgements
This work was financially supported by the National 973 Program of China (2009CB220100).Notes and references
- B. A. Boukamp, G. C. Lesh and R. A. Huggins, J. Electrochem. Soc., 1981, 128, 725–729 CrossRef CAS.
- Y. He, X. Q. Yu, Y. H. Wang, H. Li and X. J. Huang, Adv. Mater., 2011, 23, 4938–4941 CrossRef CAS PubMed.
- D. S. Jung, T. H. Hwang, S. B. Park and J. W. Choi, Nano Lett., 2013, 13, 2092–2097 CrossRef CAS PubMed.
- C. K. Chan, H. L. Peng, G. Liu, K. McIlwrath, X. F. Zhang, R. A. Huggins and Y. Cui, Nat. Nanotechnol., 2008, 3, 31–35 CrossRef CAS PubMed.
- L. F. Cui, R. Ruffo, C. K. Chan, H. L. Peng and Y. Cui, Nano Lett., 2009, 9, 491–495 CrossRef CAS PubMed.
- M. H. Park, M. G. Kim, J. Joo, K. Kim, J. Kim, S. Ahn, Y. Cui and J. Cho, Nano Lett., 2009, 9, 3844–3847 CrossRef CAS PubMed.
- T. Song, J. L. Xia, J. H. Lee, D. H. Lee, M. S. Kwon, J. M. Choi, J. Wu, S. K. Doo, H. Chang, W. Il Park, D. S. Zang, H. Kim, Y. G. Huang, K. C. Hwang, J. A. Rogers and U. Paik, Nano Lett., 2010, 10, 1710–1716 CrossRef CAS PubMed.
- J. P. Maranchi, A. F. Hepp and P. N. Kumta, Electrochem. Solid-State Lett., 2003, 6, A198–A201 CrossRef CAS.
- Y. Yu, L. Gu, C. Zhu, S. Tsukimoto, P. A. van Aken and J. Maier, Adv. Mater., 2010, 22, 2247–2250 CrossRef CAS PubMed.
- H. Jia, P. Gao, J. Yang, J. Wang, Y. Nuli and Z. Yang, Adv. Energy Mater., 2011, 1036–1039 CrossRef CAS.
- M. Thakur, R. B. Pernites, N. Nitta, M. Isaacson, S. L. Sinsabaugh, M. S. Wong and S. L. Biswal, Chem. Mater., 2012, 24, 2998–3003 CrossRef CAS.
- M.-S. Wang, L.-Z. Fan, M. Huang, J. Li and X. Qu, J. Power Sources, 2012, 219, 29–35 CrossRef CAS.
- F.-H. Du, K.-X. Wang, W. Fu, P.-F. Gao, J.-F. Wang, J. Yang and J.-S. Chen, J. Mater. Chem. A, 2013, 1, 13648–13654 CAS.
- A. Esmanski and G. A. Ozin, Adv. Funct. Mater., 2009, 19, 1999–2010 CrossRef CAS.
- Y. Yao, M. T. McDowell, I. Ryu, H. Wu, N. Liu, L. Hu, W. D. Nix and Y. Cui, Nano Lett., 2011, 11, 2949–2954 CrossRef CAS PubMed.
- B. M. Bang, H. Kim, H. K. Song, J. Cho and S. Park, Energy Environ. Sci., 2011, 4, 5013–5019 CAS.
- B. M. Bang, J. I. Lee, H. Kim, J. Cho and S. Park, Adv. Energy Mater., 2012, 2, 878–883 CrossRef CAS.
- R. Yi, F. Dai, M. L. Gordin, S. Chen and D. Wang, Adv. Energy Mater., 2013, 3, 295–300 CrossRef CAS.
- L. Shen, X. Guo, X. Fang, Z. Wang and L. Chen, J. Power Sources, 2012, 213, 229–232 CrossRef CAS.
- J. Bonse, K. W. Brzezinka and A. J. Meixner, Appl. Surf. Sci., 2004, 221, 215–230 CrossRef CAS.
- N. A. Liu, K. F. Huo, M. T. McDowell, J. Zhao and Y. Cui, Sci. Rep., 2013, 3, 1919–1925 Search PubMed.
| This journal is © The Royal Society of Chemistry 2014 |