Effect of surface chemistry of polyethyleneimine-grafted polypropylene fiber on its CO2 adsorption
Qinghua Wua,
Shuixia Chen*ab and
Hui Liub
aPCFM Lab, School of Chemistry and Chemical Engineering, Sun Yat-Sen University, Guangzhou 510275, P.R. China. E-mail: cescsx@mail.sysu.edu.cn; Fax: +86-20-84034027
bMaterials Science Institute, Sun Yat-Sen University, Guangzhou 510275, P.R. China
First published on 28th May 2014
Abstract
A high amino density of grafted solid amine adsorption fiber (PP-AM-PEI) for CO2 adsorption was prepared by grafting acrylamide (AM) onto the surface of a polypropylene (PP) fiber and subsequently modified with polyethylenimine (PEI). The grafting of AM on PP fibers was beneficial for introducing and assembling PEI onto the fibers. An examination by infrared and scanning electron microscopies confirmed that AM and PEI were successfully introduced onto the surface of PP fiber. PP-AM-PEI showed good thermo-stability, a high adsorption capacity for CO2 (5.91 mmol g−1). It also has good regeneration performance and could maintain almost the same adsorption capacity for CO2 after 5 recycle numbers. In addition, two other polypropylene-based adsorbents, PP-GMA-PEI (using glycidyl methacrylate (GMA) as the active monomer) and PP-AM-EDA (using ethylenediamine (EDA) as the functional monomer) were prepared. Compared with PP-GMA-EDA, PP-AM-PEI was more suitable for CO2 capture in the presence of moisture due to its hydrophilicity. The results also showed that the adsorption capacity of PP-AM-PEI was superior to PP-AM-EDA due to the high amino density of PEI.
1. Introduction
Global warming is a huge challenge for mankind in the 21st century. CO2, which mostly comes from flue gas, has been identified as a critical component of greenhouse gases. To reduce CO2 emissions and its negative impact on the environment, a large number of effective materials1–3 and technologies4–7 have been researched. Among the different types of materials, liquid amine absorbents8–10 are widely used, owing to their higher selectivity and absorption capacity for removing CO2 from flue gas. However, there are some unavoidable issues such as viscosity, excessive corrosion equipment, high energy consumption and foaming that severely hindered its wide application. Thus, it is necessary to develop a promising alternative adsorbent, which has higher adsorption selectivity, larger adsorption capacity and better regeneration ability, but lower danger during transport and storage. Fortunately, solid adsorption materials11 can meet these requirements.Solid adsorption materials, particularly porous silica-supported amine materials,12–16 which can adsorb CO2 by physical and chemical adsorption,17 display outstanding adsorption properties. There are two main methods for preparing this array of silica-supported amine materials. One method is known as wet impregnation, which can introduce liquid amines like polyethylenimine (PEI)12,18 and tetraethylenepentamine (TEPA)19 directly into the porous silica supports by physical impregnation. The other method uses chemical grafting to combine amines and supports.20 Compared with the former, the latter can not only introduce more different types of amines onto the supports, but can also avoid amine leaking and agglomeration,21 which can endow the material with higher stability. In both methods, PEI was used on a large scale because it contains abundant primary, secondary and tertiary amines. Therefore, by employing PEI, the CO2 adsorption capacity of the porous silica supports can be significantly improved. For porous silica supports, the pore volume22 and pore structure23 are favorable to enhance the CO2 adsorption capacity. However, large amount of amine loading on these supports, particularly microporous and mesoporous silica supports may cause serious pore blockage and CO2 diffusion resistance, leading to the reduction of adsorption capacity.
Compared with porous silica supports, the adsorption capacity of fibrous adsorbent is unaffected by pore volume and pore diameter. For one thing, it can be prepared in various forms (including films, filters, nonwoven fabric, etc.), for another, it has large external surface area, short transit distance, low pressure drops, high chemical stability and desirable flexibility. Besides, grafting has little influence on the surface areas of the fibers, which can effectively improve their adsorption capacity for CO2, even with high grafting rate.24 Hence, solid amine fibrous adsorbents will have great promise in future applications.
In this study, polypropylene (PP) fiber was employed as a support. By combining graft polymerization with chemical modification on the surface of the PP fiber, a novel solid amine fiber (PP-AM-PEI) for CO2 adsorption was prepared. The PP fiber was functionalized via covalent binding with PEI, which is rich in amines and exhibits an outstanding adsorption ability for CO2. The effect of the grafting monomer, adsorption performance and regeneration ability of PP-AM-PEI were studied.
2. Experimental section
2.1 Materials and reagents
All reagents were purchased as analytical grade (AR) and used without further purification, except glycidyl methacrylate (GMA). The inhibitor in the glycidyl methacrylate monomer was removed with neutral aluminum oxide before use. Polypropylene (PP) fibers were provided by Xinshun Special Fiber Company (Zhongshan, China). Branched polyethylenimine (PEI, Mw = 600; 1800; 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000; and 70
000; and 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) was purchased from Aladdin. The others, including acrylamide (AM) and ammonium ferrous sulfate [(NH4)2SO4·FeSO4·6H2O], were purchased from Tianjin Fuchen Chemical Reagents Factory.
000) was purchased from Aladdin. The others, including acrylamide (AM) and ammonium ferrous sulfate [(NH4)2SO4·FeSO4·6H2O], were purchased from Tianjin Fuchen Chemical Reagents Factory.
2.2 Preparation of PP-AM-PEI
The preparation process of PP-AM-PEI was illustrated in Scheme 1. First, raw PP was subjected to γ-ray irradiation at a dosage rate of 0.837 kGy h−1 to obtain preirradiated PP fibers. Then, AM was grafted onto PP fibers according to the following procedure: 85 g of H2O and 0.08 g (NH4)2SO4·FeSO4·6H2O were put in a 100 mL three-necked flask, and the oxygen in the solution was removed by purging nitrogen for 30 min. Further, 1.0 g PP and 15 g AM were introduced into the flask, and the grafting reaction was carried out at 70 °C for 2 h. After the grafting step, the fiber was washed several times with boiling deionized water to completely remove the residual monomer and homopolymers. Afterward, the grafted product PP-AM was dried in a vacuum at 60 °C for 24 h. The grafting degree of PP-AM was calculated using the following eqn (1):
 | (1) |
PEI was introduced onto PP-AM fibers by reacting with PEI (10 wt% aqueous solution) for 6 h. The obtained fiber PP-AM-PEI was rinsed with deionized water and ethanol, followed by drying under vacuum at 60 °C for 24 h. The grafting degree of PEI on PP-AM-PEI was calculated according to the following equation:
 | (2) |
Nitrogen content (N, mmol g−1) of PP-AM-PEI (amide excluded) was calculated by the equation
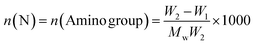 | (3) |
2.3 Preparation of grafted solid amine fibers with different chemical structures
In order to investigate relationships between structure and CO2 capture performance, a number of grafted solid amine fibers with different chemical structures were designed and prepared. The solid amine fibers (PP-x-y) were fabricated by grafting different monomers (x) and subsequently reacting with different types of amines (y) using a process similar to that of PP-AM-PEI. All materials and structures were listed in Table 1.2.4 Physical and chemical characterization
Infrared spectra in the range of 700 to 4000 cm−1 were obtained with an FT-IR analyzer (Tensor-27, Germany) equipped with a continuum microscope and an attenuated total reflection (ATR) objective.A thermogravimetric (TG) analyzer (Netzch TG-209C) was employed to determine the thermal stability of PP, PP-AM and PP-AM-PEI. The tests were carried out under a nitrogen atmosphere by heating the fiber from ambient temperature to 600 °C, with a heating rate of 10 °C min−1.
Elemental analyses (EA) were employed to determine the composition of the fibers. The nitrogen, carbon and hydrogen contents were determined by an Analysensysteme GmbH ElementarVario EL (Germany).
Fabric moisture transmission instrument model YG (B) 216-II (China) was employed to measure the hygroscopic rate of samples.
The morphology and diameter of the fiber samples were observed by an ultra-depth three-dimensional microscope (VHX-1000C, Japan).
2.5 CO2 adsorption experiment
Breakthrough curves were used to characterize the CO2 adsorption performances of all samples. Approximately 1.00 g fiber sample was tightly placed in an adsorption column (Φ = 1.3 cm), into which a dry nitrogen flow was introduced at a flow rate of 30 mL min−1 for 0.5 h to remove the air and excess water in the tube. Then, the dry CO2/N2 mixture gas was introduced through the tube at a flow rate of 30 mL min−1. The flow rate of the gas was controlled by electronic mass-control instruments. The inlet/outlet concentration of CO2 was analyzed at 2 min intervals, using a Techcomp 7900 gas chromatograph with a thermal-conductivity detector (TCD). The effect of adsorption temperature on the adsorption was investigated in the range of 25 to 75 °C. The corresponding adsorption temperature was controlled by a water bath. After adsorption, pure nitrogen gas at a flow rate of 30 mL min−1 was introduced through the tube at 85 °C to regenerate the spent fibers.3. Results and discussion
3.1 Chemical structure and the thermal stability of PP-AM-PEI
ATR-FTIR was employed to evaluate a two-step grafting process. Fig. 1 shows FTIR spectra of PP, PP-AM and PP-AM-PEI, respectively. Compared with the PP fiber, the PP-AM fiber was characterized by the peaks at 1653 cm−1 (ref. 25) and 1606 cm−1 corresponding to the stretching vibrations of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, –NH and –C–N–, which represented the existence of amide bonds. Two broad adsorption peaks at 3000–3500 cm−1, which could be attributed to primary amine, were also observed. After the grafting reaction of the PP-AM fiber with PEI, the N–H bond of primary amine, symmetric and asymmetric stretching vibrations of –CH2– at 3000–3500 cm−1, 2820 cm−1 and 2928 cm−1, respectively, all became much stronger, whereas the disappearance of two absorption peaks at 3000–3500 cm−1 corresponding to the primary amine of amide and the emerging of the absorption peak at 1573 cm−1 and to the bending of secondary amines (–N(R)H) in PEI25,26 testified the substituting PEI for amino group in amide. Briefly, the presence of these new bands in the FTIR spectra confirms the success of the grafting process.
O, –NH and –C–N–, which represented the existence of amide bonds. Two broad adsorption peaks at 3000–3500 cm−1, which could be attributed to primary amine, were also observed. After the grafting reaction of the PP-AM fiber with PEI, the N–H bond of primary amine, symmetric and asymmetric stretching vibrations of –CH2– at 3000–3500 cm−1, 2820 cm−1 and 2928 cm−1, respectively, all became much stronger, whereas the disappearance of two absorption peaks at 3000–3500 cm−1 corresponding to the primary amine of amide and the emerging of the absorption peak at 1573 cm−1 and to the bending of secondary amines (–N(R)H) in PEI25,26 testified the substituting PEI for amino group in amide. Briefly, the presence of these new bands in the FTIR spectra confirms the success of the grafting process.
The thermogravimetric analysis results of PP, PP-AM and PP-AM-PEI were shown in Fig. 2. It was found that the PP fiber began to lose its weight when the temperature was raised to 380 °C, and it showed only one platform at a mass loss of almost 100%. In the case of PP-AM, there were two platforms whose starting decomposition temperatures were 250 and 380 °C, respectively. It was believed that the first platform resulted from the degradation of the grafted AM, and the degradation of its inner PP substrate led to another loss when the temperature reached 380 °C. For PP-AM-PEI, its weight loss below 100 °C and between 100 and 200 °C could be attributed to the desorption of physically adsorbed and chemically adsorbed water,27,28 respectively. The weight losses in the temperature range from 200 °C to 380 °C could be attributed to grafted PEI and PAM; the weight losses in the temperature range from 380 °C to 500 °C could be attributed to PP. The TG results indicated that PP-AM-PEI fiber was stable when it was used at a temperature below 200 °C.
3.2 Adsorption properties of PP-AM-PEI for CO2
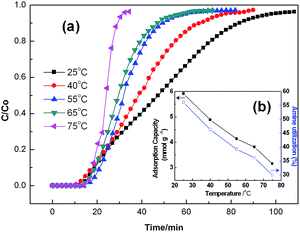
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000; and 70
000; and 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) were grafted onto the fibers (70 wt% PEI grafting), and their adsorption capacities were compared. The relevant results of these adsorbents are depicted in Fig. 3 and Table 2. As shown in Fig. 3, with the increase in PEI molecular weight, the CO2 sorption capacity increases as well. It is clear that the adsorbent with a PEI Mw of 70 K showed the best sorption capacity among all the samples, which was approximately 33% higher than that of the lowest molecular PEI (Mw = 600, 4.12 mmol g−1). In light of the elemental analysis data that the contents of N and H of the adsorbent with a PEI Mw of 70 K were the highest among the adsorbents, although their grafting degrees of PEI were the same, it is not difficult to understand why it had the highest adsorption capacity. Moreover, after the grafting of PEI, the content of N and H on PP-AM-PEI were higher than that on PP-AM, thereby providing further evidence of the successful functionalization with PEI. However, two of them on PP-AM-PEI with different molecular weights were slightly different. According to Scheme 1, the expected product generation was accompanied by the removal of ammonia. Accordingly, it can be inferred that the reaction process between the amide groups and a higher molecular weight of PEI was unlike that with the lower because the greater steric hindrance of the side chain of the higher molecular weight of PEI, the less the reactive chance.
000) were grafted onto the fibers (70 wt% PEI grafting), and their adsorption capacities were compared. The relevant results of these adsorbents are depicted in Fig. 3 and Table 2. As shown in Fig. 3, with the increase in PEI molecular weight, the CO2 sorption capacity increases as well. It is clear that the adsorbent with a PEI Mw of 70 K showed the best sorption capacity among all the samples, which was approximately 33% higher than that of the lowest molecular PEI (Mw = 600, 4.12 mmol g−1). In light of the elemental analysis data that the contents of N and H of the adsorbent with a PEI Mw of 70 K were the highest among the adsorbents, although their grafting degrees of PEI were the same, it is not difficult to understand why it had the highest adsorption capacity. Moreover, after the grafting of PEI, the content of N and H on PP-AM-PEI were higher than that on PP-AM, thereby providing further evidence of the successful functionalization with PEI. However, two of them on PP-AM-PEI with different molecular weights were slightly different. According to Scheme 1, the expected product generation was accompanied by the removal of ammonia. Accordingly, it can be inferred that the reaction process between the amide groups and a higher molecular weight of PEI was unlike that with the lower because the greater steric hindrance of the side chain of the higher molecular weight of PEI, the less the reactive chance.
| Fiber | Elemental composition (wt%) | ||
|---|---|---|---|
| C (%) | H (%) | N (%) | |
| PP | 84.30 | 13.66 | 0.00 |
| PP-AM (G = 360%) | 53.16 | 9.16 | 13.27 |
| PP-AM-PEI (PEI M.W. 600) | 45.47 | 9.72 | 15.25 |
| PP-AM-PEI (PEI M.W. 1800) | 49.49 | 10.56 | 15.00 |
| PP-AM-PEI (PEI M.W. 10 k) | 46.60 | 10.77 | 18.64 |
| PP-AM-PEI (PEI M.W. 70 K) | 48.31 | 11.01 | 18.32 |
| RNH2 + H2O ⇔ RNH3+ + OH− | (4) |
| CO2 + OH− ⇔ HCO3− | (5) |
| RNH3++HCO3− ⇔ RNH3+HCO3− | (6) |
 | ||
| Fig. 6 Breakthrough curves of PP-AM-PEI and PP-GMA-PEI. (fiber mass: 1.0 g; adsorption temperature: 25 °C; gas flow rate: 30 mL min−1). | ||
| Temperature (°C) | Amino content (mmol g−1) | Hygroscopic rate (%) | Adsorption capacity (mmol g−1) | Amine utilization (%) | |
|---|---|---|---|---|---|
| PP-AM-PEI (G = 63%) | 25 | 8.99 | 55.08 ± 1.5 | 4.8 | 53.39 |
| 40 | 3.56 | 39.6 | |||
| 65 | 2.96 | 32.91 | |||
| PP-GMA-PEI (G = 68%) | 25 | 9.41 | 20.38 ± 0.11 | 2.51 | 26.9 |
| 40 | 1.56 | 16.58 | |||
| 65 | 1.26 | 13.39 |
It is noteworthy that under moist conditions, the adsorption capacity and amine utilization (here, amine utilization is defined as the percentage of the actual number of amino for CO2 adsorption in the total number of amino) of PP-AM-PEI were both far higher than those of PP-GMA-PEI by more than 50%, although the grafting amount of PEI was nearly equal. Hygroscopic rate tests indicated PP-AM-PEI (55.08%) was more hydrophilic than PP-GMA-PEI (20.38%) after grafting modification. Hydrophilism of the fiber was markedly enhanced by the hydrophilic nature of AM, whereas less obviously by the hydrophobic monomer GMA. Therefore, it was assumed that CO2 adsorption with PP-AM-PEI, under moist conditions, was better facilitated by stronger moisture promoting effects.
Moreover, swelling experiments demonstrated that PP-AM-PEI was easier to swell than PP-GMA-PEI. As can be seen in Fig. 7, the swelling ability of the adsorbent was also distinctly promoted by the nature of AM in water, leading to more propitious opportunity for the diffusion of CO2 on the surface of PP-AM-PEI, eventually. In contrast, with the increase in temperature, their adsorption capacities and amine utilization declined, but the decrease rate of PP-GMA-PEI was faster than that of PP-AM-PEI. This could be ascribed to that for PP-GMA-PEI, which was more hydrophobic, water molecules are more inclined to be in the form of gas at high temperatures. In summary, using AM, instead of GMA, as the grafting agent can significantly improve the CO2 adsorption capacity of the PEI-grafting fiber under moist conditions.
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), which reduce the alkalinity of amine, leading to the significantly lower reactivity between amine and CO2. Despite this, a small amount of CO2 was still captured by PP-AM, which might be due to the affinity generated by physical adsorption such as electrostatic interaction, Van der Waals force, and hydrogen bonding, rather than chemisorption. It is assumed that both EDA and PEI consumed one active prime amine to react with one amide of PP-AM along with a new amide generated. Therefore, the amides should not be treated as effective amines for EDA, which means its amine utilization for CO2 capture must be calculated by using half of the total amino groups. However, in the case of PEI, as it contains all primary, secondary and tertiary amino groups, and it is difficult to clarify the contents of different amino groups reacted onto fibers. Therefore, two limiting cases are assumed: one is that only primary amines exist on the PEI-grafted adsorbent, whose repeating unit is –CH2–CH2–NH2 and its Mw is 44 (g mol−1) and the other is that only tertiary amines present on the PEI-grafted adsorbent, whose repeating unit is
O), which reduce the alkalinity of amine, leading to the significantly lower reactivity between amine and CO2. Despite this, a small amount of CO2 was still captured by PP-AM, which might be due to the affinity generated by physical adsorption such as electrostatic interaction, Van der Waals force, and hydrogen bonding, rather than chemisorption. It is assumed that both EDA and PEI consumed one active prime amine to react with one amide of PP-AM along with a new amide generated. Therefore, the amides should not be treated as effective amines for EDA, which means its amine utilization for CO2 capture must be calculated by using half of the total amino groups. However, in the case of PEI, as it contains all primary, secondary and tertiary amino groups, and it is difficult to clarify the contents of different amino groups reacted onto fibers. Therefore, two limiting cases are assumed: one is that only primary amines exist on the PEI-grafted adsorbent, whose repeating unit is –CH2–CH2–NH2 and its Mw is 44 (g mol−1) and the other is that only tertiary amines present on the PEI-grafted adsorbent, whose repeating unit is 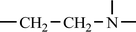 and its Mw is 42 (g mol−1). Using the two Mw limiting values to further calculate the N and amino group content, the range of 10.31–10.80 mmol g−1 can be obtained, close to that calculated by using Mw of 43 of the –CH2–CH2–NH– (10.55 mmol g−1). Therefore, it is reasonable to employ the intermediate value 43 as the Mw of the repeating unit to analyze the adsorption properties of PP-AM-PEI, which could significantly simplify the analysis process. Based on the above-mentioned analysis, the related results are illustrated in Fig. 8(a) and Table 4, which show that the saturated adsorption time decreased with the increase in temperature for both PP-AM-EDA and PP-AM-PEI. However, compared with PP-AM-EDA, it took longer for PP-AM-PEI to approach the saturated adsorption at 25 or 65 °C. Meanwhile, the amine utilization of PP-AM-EDA can reach up to 91.11%, but for PP-AM-PEI, it was just 56.01%. Moreover, under the same conditions, both the adsorption rate and the amine utilization of PP-AM-EDA were higher than those of PP-AM-PEI, because of the lower mass transfer resistance of CO2 on the surface of the former than that of the latter. Compared to the results at room temperature, the adsorption rate and the amine utilization of PP-AM-EDA decreased dramatically at 65 °C. Inversely, PP-AM-PEI can still maintain a fair adsorption rate and amine utilization at higher temperatures because the increase in temperature could drive up a higher mobility of PEI and significantly increase the total number of accessible sorption amine sites. Therefore, from what has been discussed above, we may safely draw the conclusion that the high density of amino adsorbent is more suitable for adsorption at high temperatures and in the presence of water.
and its Mw is 42 (g mol−1). Using the two Mw limiting values to further calculate the N and amino group content, the range of 10.31–10.80 mmol g−1 can be obtained, close to that calculated by using Mw of 43 of the –CH2–CH2–NH– (10.55 mmol g−1). Therefore, it is reasonable to employ the intermediate value 43 as the Mw of the repeating unit to analyze the adsorption properties of PP-AM-PEI, which could significantly simplify the analysis process. Based on the above-mentioned analysis, the related results are illustrated in Fig. 8(a) and Table 4, which show that the saturated adsorption time decreased with the increase in temperature for both PP-AM-EDA and PP-AM-PEI. However, compared with PP-AM-EDA, it took longer for PP-AM-PEI to approach the saturated adsorption at 25 or 65 °C. Meanwhile, the amine utilization of PP-AM-EDA can reach up to 91.11%, but for PP-AM-PEI, it was just 56.01%. Moreover, under the same conditions, both the adsorption rate and the amine utilization of PP-AM-EDA were higher than those of PP-AM-PEI, because of the lower mass transfer resistance of CO2 on the surface of the former than that of the latter. Compared to the results at room temperature, the adsorption rate and the amine utilization of PP-AM-EDA decreased dramatically at 65 °C. Inversely, PP-AM-PEI can still maintain a fair adsorption rate and amine utilization at higher temperatures because the increase in temperature could drive up a higher mobility of PEI and significantly increase the total number of accessible sorption amine sites. Therefore, from what has been discussed above, we may safely draw the conclusion that the high density of amino adsorbent is more suitable for adsorption at high temperatures and in the presence of water.
| Temperature (°C) | Effective amino content (mmol g−1) | Adsorption capacity (mmol g−1) | Amine utilization (%) | |
|---|---|---|---|---|
| PP-AM-PEI | 25 | 10.55 | 5.91 | 56.01 |
| 65 | 3.81 | 36.11 | ||
| PP-AM-EDA | 25 | 4.95 | 4.51 | 91.11 |
| 65 | 2.77 | 55.95 |
4. Regeneration performance
To satisfy the demands of practical use, the adsorbent should possess excellent chemical stability and regenerability for moderate desorption and long-term cyclic operation. In order to measure the stability of PP-AM-PEI, 5 cycles of adsorption-desorption were carried out, and the results are shown in Fig. 9(b). After 5 cycles, no significant change in CO2 sorption capacity was observed: the adsorption capacity of adsorbent decreased by just 8%. Furthermore, the breakthrough curves of fresh and cycle 4 were displayed in Fig. 9(a). It was evident that the regenerated fibers exhibited nearly the same adsorption behaviour as the fresh ones. All these results confirmed that the solid amine adsorbent PP-AM-PEI could keep good stability after multiple regeneration cycles and maintain its adsorption capacity for CO2.The FT-IR spectra of the fresh PP-AM-PEI, the PP-AM-PEI fiber after adsorption of CO2 and the regenerated fiber are illustrated in Fig. 10. It was evident that compared with fresh fiber, the N–H adsorption bands of adsorbed PP-AM-PEI at 3000–3500 cm−1 became significantly weaker, which could be attributed to the adsorption of CO2 onto amine. The N–H and C–H bands of fresh and regenerated PP-AM-PEI showed nearly identical IR intensity. The weight of the sample after regeneration was close to that before adsorption (with a deviation of less than 0.5%), indicating that CO2 was completely released from the PP-AM-PEI fiber after the desorption process, and the grafting PEI stayed intact during the CO2 adsorption-desorption cycles.
5. Conclusion
This study has shown that PEI-grafted polypropylene fiber could effectively remove CO2 from a simulated flue gas containing 10% CO2 at ambient conditions. The amount of PEI grafting on PP-AM has a significant influence on the adsorption performance of the adsorbents. A grafting of 83 wt% PEI shows the largest adsorption capacity of 5.91 mmol g−1 at 25 °C. It is found that a decrease in temperature favors the CO2 adsorption of the grafted PEI fibers. The correlation between the CO2 sorption capacity and the grafting monomer indicates that AM, a hydrophilic monomer, can endow the adsorbent with excellent swelling ability, which can improve the adsorption capacity during the adsorption process in the presence of water. As for the effect exerted by the amine structure, results of the amine utilization suggested that PEI was more effective and efficient to adsorb CO2 molecules due to the stretching of wrapped chain at high temperatures. Furthermore, the adsorbent can be easily and completely regenerated under mild conditions and is stable in the cyclic operations for 5 cycles. Besides, the IR studies have suggested that the PP-AM-PEI can maintain good regenerability and stability.Acknowledgements
The authors gratefully acknowledge the financial support provided by the National Natural Science Foundation of China (Grant no. 51173211), Science and Technology Project of Guangdong Province (2011B090400030), and the Science and Technology Project of Zhuhai (2010B050102024).References
- J. E. Bara, D. E. Camper, D. L. Gin and R. D. Noble, Acc. Chem. Res., 2010, 43, 152–159 CrossRef CAS PubMed.
- S. S. Razavi, S. M. Hashemianzadeh and H. Karimi, J. Mol. Model., 2011, 17, 1163–1172 CrossRef CAS PubMed.
- Y. X. Zhao, M. Seredych, Q. Zhong and T. J. Bandosz, ACS Appl. Mater. Interfaces, 2013, 5, 4951–4959 CAS.
- D. M. D'Alessandro, B. Smit and J. R. Long, Angew. Chem., Int. Ed., 2010, 49, 6058–6082 CrossRef PubMed.
- N. MacDowell, N. Florin, A. Buchard, J. Hallett, A. Galindo, G. Jackson, C. S. Adjiman, C. K. Williams, N. Shah and P. Fennell, Energy Environ. Sci., 2010, 3, 1645–1669 CAS.
- A. Sayari, Y. Belmabkhout and R. Serna-Guerrero, Chem. Eng. J., 2011, 171, 760–774 CrossRef CAS PubMed.
- J. Schrier, ACS Appl. Mater. Interfaces, 2012, 4, 3745–3752 CAS.
- N. Harun, T. Nittaya, P. L. Douglas, E. Croiset and L. A. Ricardez-Sandoval, Int. J. Greenhouse Gas Control, 2012, 10, 295–309 CrossRef CAS PubMed.
- G. Richner and G. Puxty, Ind. Eng. Chem. Res., 2012, 51, 14317–14324 CrossRef CAS.
- J. Tan, H. W. Shao, J. H. Xu, L. Du and G. S. Luo, Ind. Eng. Chem. Res., 2011, 50, 3966–3976 CrossRef CAS.
- Q. A. Wang, J. Z. Luo, Z. Y. Zhong and A. Borgna, Energy Environ. Sci., 2011, 4, 42–55 CAS.
- X. C. Xu, C. S. Song, J. M. Andresen, B. G. Miller and A. W. Scaroni, Energy Fuels, 2002, 16, 1463–1469 CrossRef CAS.
- X. C. Xu, C. S. Song, J. M. Andresen, B. G. Miller and A. W. Scaroni, Microporous Mesoporous Mater., 2003, 62, 29–45 CrossRef CAS.
- J. C. Hicks, J. H. Drese, D. J. Fauth, M. L. Gray, G. G. Qi and C. W. Jones, J. Am. Chem. Soc., 2008, 130, 2902–2903 CrossRef CAS PubMed.
- X. L. Ma, X. X. Wang and C. S. Song, J. Am. Chem. Soc., 2009, 131, 5777–5783 CrossRef CAS PubMed.
- A. Goeppert, M. Czaun, R. B. May, G. K. S. Prakash, G. A. Olah and S. R. Narayanan, J. Am. Chem. Soc., 2011, 133, 20164–20167 CrossRef CAS PubMed.
- R. Serna-Guerrero, E. Da'na and A. Sayari, Ind. Eng. Chem. Res., 2008, 47, 9406–9412 CrossRef CAS.
- X. X. Wang, X. L. Ma, C. S. Song, D. R. Locke, S. Siefert, R. E. Winans, J. Mollmer, M. Lange, A. Moller and R. Glaser, Microporous Mesoporous Mater., 2013, 169, 103–111 CrossRef CAS PubMed.
- X. R. Wang, H. Q. Li, H. T. Liu and X. J. Hou, Microporous Mesoporous Mater., 2011, 142, 564–569 CrossRef CAS PubMed.
- J. H. Drese, S. Choi, R. P. Lively, W. J. Koros, D. J. Fauth, M. L. Gray and C. W. Jones, Adv. Funct. Mater., 2009, 19, 3821–3832 CrossRef CAS.
- Z. H. Zhang, X. L. Ma, D. X. Wang, C. S. Song and Y. G. Wang, AIChE J., 2012, 58, 2495–2502 CrossRef CAS.
- D. J. Fauth, M. L. Gray, H. W. Pennline, H. M. Krutka, S. Sjostrom and A. M. Ault, Energy Fuels, 2012, 26, 2483–2496 CrossRef CAS.
- X. L. Yan, L. Zhang, Y. Zhang, G. D. Yang and Z. F. Yan, Ind. Eng. Chem. Res., 2011, 50, 3220–3226 CrossRef CAS.
- Y. Yang, H. C. Li, S. X. Chen, Y. N. Zhao and Q. H. Li, Langmuir, 2010, 26, 13897–13902 CrossRef CAS PubMed.
- B. Gao, H. Lei, L. Jiang and Y. Zhu, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2007, 853, 62–69 CrossRef CAS PubMed.
- Q. J. Chen, F. C. Fan, D. H. Long, X. J. Liu, X. Y. Liang, W. M. Qiao and L. C. Ling, Ind. Eng. Chem. Res., 2010, 49, 11408–11414 CrossRef CAS.
- L. F. Wang and R. T. Yang, J. Phys. Chem. C, 2011, 115, 21264–21272 CAS.
- S. Loganathan, M. Tikmani and A. K. Ghoshal, Langmuir, 2013, 29, 3491–3499 CrossRef CAS PubMed.
- P. Y. Li, B. Q. Ge, S. J. Zhang, S. X. Chen, Q. K. Zhang and Y. N. Zhao, Langmuir, 2008, 24, 6567–6574 CrossRef CAS PubMed.
- A. Heydari-Gorji and A. Sayari, Chem. Eng. J., 2011, 173, 72–79 CrossRef CAS PubMed.
- X. C. Xu, C. S. Song, B. G. Miller and A. W. Scaroni, Ind. Eng. Chem. Res., 2005, 44, 8113–8119 CrossRef CAS.
- P. D. Vaidya and E. Y. Kenig, Chem. Eng. Sci., 2007, 62, 7344–7350 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |