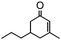
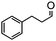
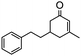
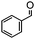
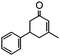
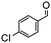
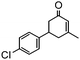
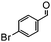
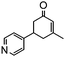
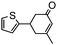
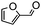
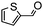The synthesis of cyclohexenone using L-proline immobilized on a silica gel catalyst by a continuous-flow approach†
Cong Zhi,
Jiaqing Wang,
Bin Luo,
Xinming Li,
Xueqin Cao,
Yue Pan and
Hongwei Gu*
Key Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, 215123, China. E-mail: hongwei@suda.edu.cn; Fax: +86-65880905; Tel: +86-65880905
First published on 14th March 2014
Abstract
A facile and convenient method for the synthesis of cyclohexenone compounds was developed using an L-proline immobilized silica gel catalyst combined with a continuous-flow approach. Because of the mild reaction conditions, ease of catalyst recyclability, and product isolation, this reaction approach can potentially be used in a facile scale-up reaction or in industrial applications.
Cyclohexenone and cyclohexanone derivatives, as high-value molecules, serve as a common structural motif for many natural products and important intermediates of organic compounds such as dihydrojunenol, faurinone, β-cadinene and other natural products1 because of their wide range of applications in pharmaceuticals, pesticides, photographic film materials, drugs and other important chemicals.2 Traditionally, substituted cyclohexenones are prepared using the well-known Robinson annulation, which combines three reactions: Michael addition, intramolecular aldol reaction, and dehydration.3 For example, Fuchs et al. introduced a multistep process starting with enantiopure epoxyvinyl sulfones for the preparation of chiral 4-alkylcycloalkenones and enantiopure 2,5-cyclohexadienone synthons.4 Both Baran and Nicolaou's groups used Robinson annulations to produce dihydrojunenol and ent-7-epizingiberene, respectively.5 Recently, Zhao et al.6 and Lu et al.7 separately used primary–secondary diamine and primary amino acid catalysts for the generation of α, β-unsaturated ketones with outstanding results.
L-Proline and its derivatives, as well-known asymmetric organocatalysts,8 have found wide application in the catalysis of enantioselective aldol reactions9 between aldehydes and acetone for C–C bond formation and Michael reactions under homogeneous conditions.10 However, because of the inherent drawbacks of homogeneous catalysts (e.g., short catalyst lifetime, large amount of organic solvent, and difficulties in catalyst separation and recovery), as well as practical difficulties,11 heterogeneous catalysts based on L-proline immobilized on different solid supports (e.g., organic polymers,12 zeolites,13 mesoporous silica14 and iron oxide nanoparticles15) have been studied.
In addition, continuous-flow technologies have recently emerged as new strategies for the sustainable generation of fine chemicals and pharmaceuticals,16 because of a number of advantages over traditional batch procedures such as a shorter reaction time, efficient mixing, and faster heat and mass transfer.17 Although many research groups have used proline organocatalysts in continuous-flow approaches for aldol reactions,18 a number of drawbacks still exist19 such as (i) long processing times, (ii) difficulties in product separation, and (iii) low yields that restrict their applications.
Therefore, the development of a more sustainable and industrially reliable catalytic procedure for the aldol reaction is still of continuing interest and a significant challenge for synthetic chemists. Herein, we report a simple, green and efficient method for the aldol reaction by a continuous-flow approach in which L-proline was immobilized on silica gel to produce a heterogenous catalyst that triggered the aldol reaction in a silica-packed chromatographic column upon the flow of eluent. The described technique has many merits such as catalyst reclability, ease of product isolation, and mild reaction conditions.
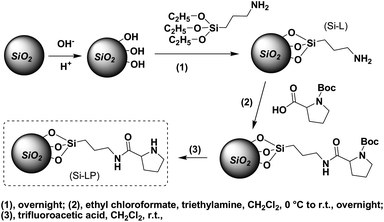
Because of its thermal and kinetic stability as well as its chemical inertness, we chose silica as a solid support for proline immobilisation. The silica gel-supported proline catalyst was prepared by following the reaction conditions and procedures reported previously20 and as illustrated in Scheme 1. These were divided into three steps. Firstly, silica gel powder was activated by sodium hydroxide to create hydroxyl groups on its surface, and it was reacted with aminopropyl triethoxysilane to afford silica gel (Si-L) with amino group modification on its surface. Upon activation by ethyl chloroformate, N-Boc-L-proline reacted with Si-L to form Si-LP (Boc). After removing the protecting groups from proline by trifluoroacetic acid (TFA) we obtained a silica gel-supported catalyst (Si-LP) as the final product. From the FTIR spectra of the Si-LP and the bare silica gel presented in Fig. S1,† we found that the two spectra had similar characteristics for most of the peaks except for three additional bands around 2832 cm−1, 1640 cm−1 and 3200 cm−1 in the spectrum of Si-LP. These come from the stretching vibrations of the –CH2–, –NHCO– and –NH-groups from proline, respectively, indicating the successful attachment of proline onto the surface of the silica gel.
The catalytic performance of Si-LP in the aldol reaction was evaluated in a model reaction between benzaldehyde and acetone, and this is shown in Scheme 2. First, the Si-LP powder (35 mL) was packed into an upright chromatographic column of 22 mm in diameter and 0.3 m in length. It was then rinsed with a sufficient amount of ethanol to remove the physically absorbed N-Boc-L-proline or L-proline on the surface of the silica gel. Then, an appropriate amount of benzaldehyde (102 μL, 1 mmol) and acetone (100 mL, 1362 mmol) was charged and eluted with dehydrated ethanol (250 mL) at room temperature and at atmospheric pressure at a flow rate of around 0.1 mL min−1. The reaction solution relied on gravity downflow. The collected solution was recharged into the chromatography column and this procedure was repeated 10 times. The resultant product mixture was subjected to GC, GC-MS and NMR analysis. The chemical structure of the product was verified by NMR spectroscopy (Fig. S3†) and the yield was about 82.1%, as determined by GC measurement (Table 1, entry 4). In the above reaction process we observed that the α, β-unsaturated ketones were generated as intermediates (Scheme 2), which further reacted with acetone to afford the final α, β-unsaturated cyclohexenones via Michael addition and Robinson cyclization.
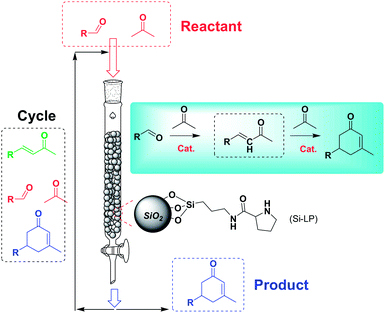 | ||
| Scheme 2 Illustration of the aldol reaction triggered by Si-LP in a silica-packed chromatographic column. | ||
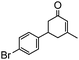
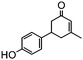
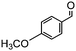
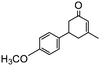
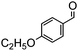
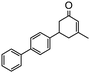
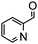
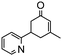
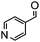
Under the conditions used for the formation of α, β-unsaturated cyclohexenone from benzaldehyde and acetone, we further examined the catalytic ability of Si-LP using other aldehyde substrates that contain aromatic, aliphatic or heterocyclic subgroups (Table 1). As shown in Table 1, almost all the aldehyde substrates were quantitatively converted to their corresponding cyclohexenone products (Table 1, entries 2–16). For example, aromatic aldehydes such as p-bromobenzaldehyde and p-cyanobenzaldehyde (Table 1, entries 6 and 7) gave the highest yields of around 99 and 97.1%, respectively (Table 1, entries 4 and 5). Other para-substituted substrates as well as benzaldehyde that contain methoxy, ethoxy, propoxy, phenyl, hydroxy or chloro groups exhibited a relatively high conversion with acceptable yields (Table 1 entries 8–12 and 5). The yields for heterocyclic aldehydes (Table 1, entries 13–16) were still attractive at 74.1% to 88.9%. In the control, when the synthesis was performed as a batch reaction, under the same conditions, the yield was obviously lower than that from the continuous-flow approach (Table S1†).
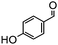
Electron-withdrawing effects have a great impact on these reactions. The substrates containing chloro, bromo or cyano groups enhanced the electron-withdrawing effects of the benzene rings, which resulted in a higher yield (Table 1, entries 5–7) compared with substrates containing methoxy, ethoxy or propoxy groups (Table 1, entries 9–11). Additionally, substrates containing obvious electron-donating groups such as formaldehyde, butyraldehyde, p-biphenylaldehyde and p-hydroxybenzaldehyde gave yields of 50.5, 55.7, 72.5 and 85.7% (Table 1, entries 1, 2, 8 and 12), respectively.
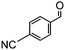
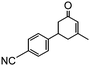
To obtain more information about the mechanism of this catalytic process, GC measurements were carried out to study the model reaction between p-cyanobenzaldehyde and acetone with Si-LP as the catalyst over time. The kinetic curve of the transformation from p-cyanobenzaldehyde to cyclohexenone is shown in Fig. 1. During the reaction, the concentration of a appeared to increase over the first 18 h and then decreased upon its conversion to the cyclohexenone compound b. The reaction is complete in 60 h. α, β-unsaturated ketone a was observed to be an intermediate in the reaction between the aldehyde and acetone, and it can further react with acetone to afford cyclohexenone b via Michael addition and Robinson cyclization.
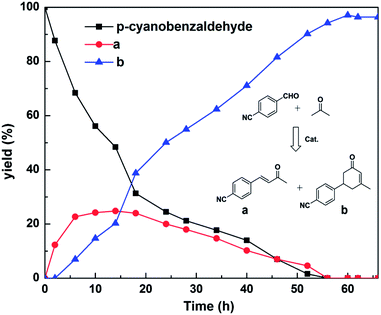 | ||
| Fig. 1 Time–conversion plots for the generation of α, β-unsaturated cyclohexenones from p-cyanobenzaldehyde and acetone with Si-LP as the catalyst. | ||
In summary, we successfully developed an efficient, environmentally friendly method for the generation of cyclohexenone using a L-proline immobilized silica gel catalyst by a continuous-flow approach. Because of the many advantages exhibited by our system such as catalyst recyclability, ease of product isolation and mild reaction conditions, this system may be a useful guideline for the design of more efficient methods of cyclohexenone production with industrial application possibilities. Further investigations into the L-proline immobilized silica gel catalyst for wider applications are ongoing in our laboratory.
H. W. G. acknowledges the financial support from NSFC (no. 21003092, no. 21373006), the Key Project of Chinese Ministry of Education (no. 211064), and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Notes and references
- (a) J. Becker and K. Bergander, Angew. Chem., Int. Ed., 2008, 47, 1654–1657 CrossRef CAS PubMed; (b) T. Findley, D. Sucunza, L. Miller, D. Davies and D. Procter, Chem. –Eur. J., 2008, 14, 6862–6865 CrossRef CAS PubMed.
- (a) S. Senguttuvan and S. Nagarajan, J. Heterocycl. Chem., 2009, 46, 1346–1348 CrossRef CAS; (b) B. D. Chong and Y. I. Ji, J. Org. Chem., 1997, 62, 9323–9325 CrossRef CAS; (c) D. B. Ramachary and K. Ramakumar, J. Org. Chem., 2007, 72, 1458–1463 CrossRef CAS PubMed.
- (a) N. Itagaki and M. Kimura, Org. Lett., 2005, 7, 4185–4188 CrossRef CAS PubMed; (b) G. Stork and A. Brizzolara, J. Am. Chem. Soc., 1963, 85, 207–222 CrossRef CAS; (c) V. H. Rawal and S. A. Kozmin, J. Am. Chem. Soc., 1999, 121, 9562–9573 CrossRef; (d) L. A. Paquette, R. Guevel, S. Sakamoto and I.-H. Kim, J. Org. Chem., 2003, 68, 6069–6107 Search PubMed; (e) Y. Inokoishi, N. Sasakura, K. Nakano, Y. Ichikawa and H. Kotsuki, Org. Lett., 2010, 12, 1616–1619 CrossRef CAS PubMed; (f) Y. K. Liu, C. Ma, K. Jiang, T. Y. Liu and Y. C. Chen, Org. Lett., 2009, 11, 2848–2851 CrossRef CAS PubMed; (g) L. Chen, S. Luo, J. Li, X. Li and J. P. Cheng, Org. Biomol. Chem., 2010, 8, 2627–2632 RSC; (h) Y. Hayashi, M. Toyoshima, H. Gotoh and H. Ishikawa, Org. Lett., 2008, 11, 45–48 CrossRef PubMed; (i) H. Yang and R. G. Carter, Org. Lett., 2010, 12, 3108–3111 CrossRef CAS PubMed; (j) M. D. Pierce, R. C. Johnston, S. Mahapatra, H. Yang, R. G. Carter and P. H. Y. Cheong, J. Am. Chem. Soc., 2012, 134, 13624–13631 CrossRef CAS PubMed; (k) T. I. Houjeiry, S. L. Poe and D. T. McQuade, Org. Lett., 2012, 14, 4394–4397 CrossRef CAS PubMed.
- J. Evarts, E. Torres and P. L. Fuchs, J. Am. Chem. Soc., 2002, 124, 11093–11101 CrossRef CAS PubMed.
- (a) K. Chen and P. S. Baran, Nature, 2009, 459, 824–828 CrossRef CAS PubMed; (b) K. C. Nicolaou and D. Sarlah, Angew. Chem., Int. Ed., 2007, 119, 4792–4795 CrossRef.
- H. F. Cui, Y. Q. Yang, Z. Chai, P. Li, C. W. Zheng, S. Z. Zhu and G. Zhao, J. Org. Chem., 2009, 75, 117–122 CrossRef PubMed.
- (a) L. W. Xu, J. Luo and Y. Lu, Chem. Commun., 2009, 14, 1807–1821 RSC; (b) L. W. Xu and Y. Lu, Org. Biomol. Chem., 2008, 6, 2047–2053 RSC.
- Handbook of asymmetric heterogeneous catalysis, ed. K. Ding and Y. Uozumi, Wiley-VCH, Germany, 2008 Search PubMed.
- (a) X. Liu, R. S. Ding, L. He, Y. M. Liu, Y. Cao, H. Y. He and K. N. Fan, ChemSusChem, 2013, 6, 604–608 CrossRef CAS PubMed; (b) J. Seayad, P. K. Patra, Y. Zhang and J. Y. Ying, Org. Lett., 2008, 10, 953–956 CrossRef CAS PubMed.
- (a) K. Sakthivel and W. Notz, J. Am. Chem. Soc., 2001, 123, 5260–5267 CrossRef CAS PubMed; (b) X. Wu and C. He, Chem. Commun., 2011, 47, 8415–8417 RSC; (c) N. Zotova and A. Franzke, J. Am. Chem. Soc., 2007, 129, 15100–15101 CrossRef CAS PubMed; (d) M. Gruttadauria and S. Riela, Tetrahedron Lett., 2004, 45, 6113–6116 CrossRef CAS; (e) B. List and L. Hoang, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 5839–5842 CrossRef CAS PubMed; (f) A. G. Doyle and E. N. Jacobsen, Chem. Rev., 2007, 107, 5713–5743 CrossRef CAS PubMed.
- (a) A. Martínez-Castañeda and H. Rodríguez-Solla, J. Org. Chem., 2012, 77, 10375–10381 CrossRef PubMed; (b) M. Gruttadauria and F. Giacalone, Chem. Soc. Rev., 2008, 37, 1666–1688 RSC; (c) R. Mestres, Green Chem., 2004, 6, 583–603 RSC; (d) G. J. Kelly and F. King, Green Chem., 2002, 4, 392–399 RSC.
- (a) K. Akagawa and S. Sakamoto, Tetrahedron Lett., 2005, 46, 8185–8187 CrossRef CAS; (b) J. Kofoed and J. Nielsen, Bioorg. Med. Chem. Lett., 2003, 13, 2445–2447 CrossRef CAS PubMed; (c) D. Font and S. Sayalero, Org. Lett., 2008, 10, 337–340 CrossRef CAS PubMed.
- (a) Microreactors in Organic Chemistry and Catalysis, ed. T. Wirth, Wiley-VCH, Singapore, 2013 Search PubMed; (b) F. Calderón and R. Fernández, Adv. Synth. Catal., 2005, 347, 1395–1403 CrossRef.
- (a) E. A. Prasetyanto and S. C. Lee, Chem. Commun., 2008, 17, 1995–1997 RSC; (b) C. Aprile and F. Giacalone, Green Chem., 2007, 9, 1328–1334 RSC.
- S. Luo and X. Zheng, Chem. Commun., 2008, 44, 5719–5721 RSC.
- (a) S. B. Ötvös, Ph.D. Dissertation, University of Szeged Institute of Pharmaceutical Chemistry, Szeged, 2013; (b) J. Wegner and S. Ceylan, Chem. Commun., 2011, 47, 4583–4592 RSC; (c) F. Lévesque and P. H. Seeberger, Org. Lett., 2011, 13, 5008–5011 CrossRef PubMed; (d) D. B. Ushakov, K. Gilmore, D. Kopetzki, D. T. McQuade and P. H. Seeberger, Angew. Chem., Int. Ed., 2014, 53, 557–561 CrossRef CAS PubMed.
- (a) J. G. Stevens and R. A. Bourne, Green Chem., 2009, 11, 409–416 RSC; (b) J. Wegner and S. Ceylan, Adv. Synth. Catal., 2012, 354, 17–57 CrossRef CAS; (c) F. E. Valera and M. Quaranta, Angew. Chem., Int. Ed., 2010, 49, 2478–2485 CrossRef CAS PubMed; (d) S. Martín, R. Porcar, E. Peris, M. I. Burguete, E. García-Verdugo and S. V. Luis, Green Chem., 2014, 16, 1639–1647 RSC.
- (a) A. Massi and A. Cavazzini, Tetrahedron Lett., 2011, 52, 619–622 CrossRef CAS; (b) O. Bortolini and A. Cavazzini, Chem. –Eur. J., 2013, 19, 7802–7808 CrossRef CAS PubMed; (c) Y. Arakawa and M. Wiesner, Adv. Synth. Catal., 2011, 353, 1201–1206 CrossRef CAS.
- (a) E. Alza and S. A. Sayalero, Chem. –Eur. J., 2009, 15, 10167–10172 CrossRef CAS PubMed; (b) N. Nikbin and P. Watts, Org. Process Res. Dev., 2004, 8, 942–944 CrossRef CAS; (c) N. T. Phan and D. H. Brown, Green Chem., 2004, 6, 526–532 RSC.
- (a) A. Zamboulis and N. J. Rahier, Tetrahedron: Asymmetry, 2009, 20, 2880–2885 CrossRef CAS; (b) B. Karimi and A. Biglari, Angew. Chem., Int. Ed., 2007, 46, 7210–7213 CrossRef CAS PubMed; (c) E. G. Doyagüez and F. Calderón, J. Org. Chem., 2007, 72, 9353–9356 CrossRef PubMed; (d) D. Dhar and I. Beadham, Proc. – Indian Acad. Sci., Chem. Sci., 2003, 115, 365–372 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures include a synthetic protocol for the construction of Si-LP, the synthesis of cyclohexenones from corresponding aldehydes. And also, the FTIR, TGA spectra of Si-LP and full spectroscopy data for all compounds. See DOI: 10.1039/c4ra01231c |
| This journal is © The Royal Society of Chemistry 2014 |