Response times of polymer dispersed liquid crystals with linear or graft copolymer matrix prepared by controlled living polymerization
Tian Lan,
Wenjie Yang,
Hao Huang and
Yinghan Wang*
State Key Laboratory of Polymer Materials Engineering, College of Polymer, Science and Engineering, Sichuan University, Chengdu 610065, PR China. E-mail: wang_yh@scu.edu.cn; Fax: +86 28 8546 0823; Tel: +86 28 8546 0823
First published on 14th March 2014
Abstract
Response time, an important property of polymer dispersed liquid crystals (PDLCs), was reported to be affected by the structures of the polymer matrix. In this study, reversible addition fragmentation chain transfer (RAFT) polymerization and atom transfer radical polymerization (ATRP) were used to synthesize a well-defined copolymer matrix. A kind of graft macroinitiator with both controlled main chain and branched chains was employed to prepare PDLCs with a graft copolymer matrix; meanwhile a linear macroinitiator was also synthesized to prepare PDLCs with a linear copolymer matrix. The effect of different matrix structures on response times was investigated. It was found that the introduction of branched chains made a big difference to response times, and a possible mechanism was proposed.
1. Introduction
Polymer dispersed liquid crystals (PDLCs), a kind of novel material, have intensively attracted researcher's interests for their particular properties.1–5 Under a suitable electric field, a PDLC film can be switched from a light-scattering opaque state (OFF state) to an optical transparent state (ON state) without polarizer and alignment layers because of the refractive index (RI) matching between liquid crystals (LCs) and the polymer matrix.6 Generally speaking, many methods have been reported to prepare PDLCs, such as thermally induced phase separation (TIPS), solvent induced phase separation (SIPS) and polymerization induced phase separation (PIPS).7 Among them, the most convenient method is PIPS because of its industrialization prospect, in which a homogeneous solution of monomer/prepolymer and liquid crystal was initiated (usually by UV light) and polymerized until the polymer and liquid crystal were well separated. Due to PDLC's unique properties, they find applications such as light shutters, smart windows and large-scale flexible displays in a range of optical devices.8 For practical applications, PDLC films with fast response times, including rise time and decay time is of great importance.It is reported that the response times of PDLC films are dependent on several parameters. Many researchers discovered that the shape of LC droplets was an important factor to affect the response times of PDLCs.9 Drzaic reported that PDLC films with large droplets had faster rise time than those with small droplets.18 Beyond that, the structure of polymer matrix is obviously an important factor to control the response times of PDLCs.13,19 Thus, a clear study of the size of LC droplets9–12 and the properties of polymer matrix13–17 on the boundary surface13 is necessary. There can be no doubt that all of these factors can affect the anchoring force between LC droplets and polymer matrix. In general, this anchoring force is the most important factor to affect the response times of PDLCs.
In our previous work, living polymerization played an important role in the preparation of PDLC films for its good properties in synthesizing well-defined polymers.20–27 But these researches were mainly focused on the electro-optical properties of PDLCs, such as driving voltage, transmittance and memory effect, seldom on the response times. On one hand, it was reported that the size of the LC droplets and the molecular weight of polymer matrix had great influence on the response times of PDLCs,9 and the different structures between linear and graft copolymer matrixes had enormous impact on the two factors.27 On the other hand, the anchoring force on the boundary surface is a factor to influence the response times,13 and the different structures between linear and graft copolymer matrixes precisely affect the anchoring force on the boundary surface. Thus, it is essential to require a better understanding of the effect of different structures between linear and graft copolymer matrixes on the response times of PDLC films.
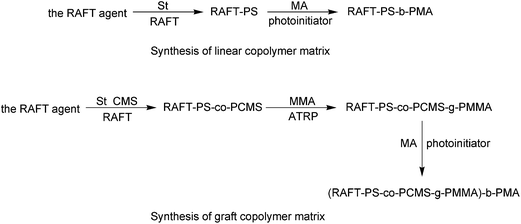
In this article, reversible additional fragmental chain transfer (RAFT) polymerization and atom transfer radical polymerization (ATRP) were employed to prepare PDLCs with well-defined copolymer matrix. Therefore, the graft macroinitiator (RAFT-PS-co-PCMS-g-PMMA) was synthesized via RAFT polymerization and ATRP. CMS was short for 4-chloromethyl styrene. Meanwhile, a linear macroinitiator (RAFT-PS) was also synthesized by RAFT polymerization, whose degree of polymerization was similar to that of the main chain of graft macroinitiator.
Based on the experimental results performed by us,27 we used Kruss DSA100 goniometer system to calculate the surface energy on the boundary surface quantificationally. The influence of different structures between linear and graft copolymer matrixes on the response times of PDLC films was investigated.
2. Experimental
2.1 Materials
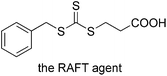
A positive dielectric anisotropy nematic liquid crystal E7 (no = 1.521, Δn = 0.22, TN–I = 60 °C) was obtained from Yantai Xian Hua Chem-Tech. Co., Ltd. Styrene (St), methyl acrylate (MA) and methyl methacrylate (MMA) (98.0%, analytical grade, from Tianjing Chemical Reagent Co., China) were passed through a column of silica to remove inhibitors. Photoinitiator (1104, from Changzhou LanDing Sci-Tech. Co., Ltd.), tetrahydrofuran (THF) (99.0%, analytical grade, from Tianjing Chemical Reagent Co., China), 2,2′-azobis(isobutyronitrile) (AIBN), N,N-dimethylformamide (DMF) (99.5%, analytical grade, from Tianjing Chemical Reagent Co., China), N,N,N′,N′′,N′′′-pentamethyldiethylenetriamine (PMDETA) (99.0%, analytical grade, from Aladdin Industrial Inc.), CuBr (99.0%, analytical grade, from Aladdin Industrial Inc.), 4-chloromethyl styrene (CMS) (99.0%, analytical grade, from WDL Chemical Reagent Co., Jiangsu) and other reagents were used as received. Trithiocarbonate (the structure was shown in Fig. 1) was synthesized according to the literature.27,282.2 Preparations of RAFT-PS, RAFT-PS-co-PCMS and RAFT-PS-co-PCMS-g-PMMA
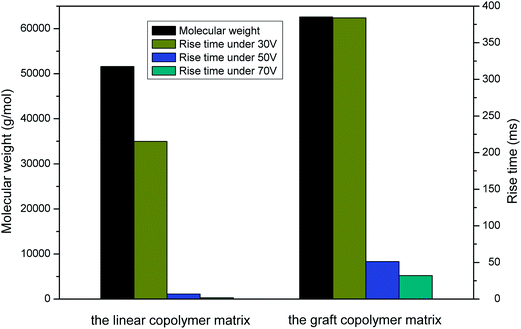
The RAFT-PS and RAFT-PS-co-PCMS were synthesized according to the literature.27 The samples were analyzed by GPC shown in Table 1.| Samples | Mn (×10−3 g mol−1) | Mw (×10−3) | PDI | |
|---|---|---|---|---|
| The linear macroinitiator | RAFT-PS | 3.96 | 4.78 | 1.21 |
| RAFT-PS-co-PCMS | 4.77 | 5.64 | 1.18 | |
| The graft macroinitiator | RAFT-PS-co-PCMS-g-PMMA | 13.9 | 19.7 | 1.41 |
| The linear copolymer matrix | RAFT-PS-b-PMA | 51.6 | 123.9 | 2.40 |
| The graft copolymer matrix | (RAFT-PS-co-PCMS-g-PMMA)-b-PMA | 62.6 | 244.4 | 3.90 |
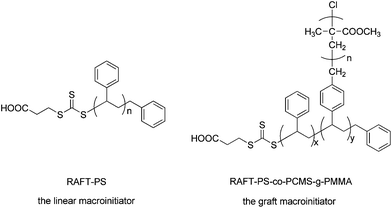
The RAFT-PS-co-PCMS-g-PMMA was synthesized according to the literature.27 (The same to the g-PMMA-1 in the literature27). The Table 1 showed the molecular weights of these macroinitiators measured by GPC. The structures of macroinitiators were shown in Fig. 2.
The synthesis was shown in Fig. 4.
2.3 Preparation of PDLCs26,27
The obtained macroinitiator (RAFT-PS or RAFT-PS-co-PCMS-g-PMMA) was dissolved in MA with predetermined ratio, and a photoactive solution was obtained. PDLCs were prepared by PIPS from a homogeneous mixture of photoactive solution, LC E7 and photoinitiator in a predetermined ratio as shown in Table 2. The reactive mixture was sandwiched between glass substrates covered with indium tin oxide (ITO). Glass spheres were used to make the thickness of the cells to be about 20 μm. Then, cells were exposed to a UV light (100 W) for 1 h. The distance was 20 cm and the temperature was about 30 °C. The wavelength of the UV light is in the range of 300–400 nm and peaked at 365 nm.| Samplea | Macroinitiator | Liquid crystal E7 (weight ratio) | Macroinitiator![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MA (number ratio) MA (number ratio) |
Photoinitiator concentrationb (wt%) |
|---|---|---|---|---|
| a All the samples were prepared by exposing to a 100 W UV light from a distance of 20 cm for 1 h at 30 °C.b The photoinitiator, 1104, was added additionally in wt% of MA. | ||||
| 1 | RAFT-PS | 50% | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 1000 |
1 |
| 2 | RAFT-PS-co-PCMS-g-MMA | 50% | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 1000 |
1 |
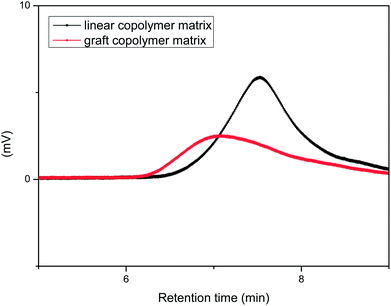
Fig. 3 showed the structures of RAFT-PS-b-PMA (linear copolymer matrix) and (RAFT-PS-co-PCMS-g-PMMA)-b-PMA (graft copolymer matrix). Simple models for the structures of theirs were also shown in Fig. 3. The synthesis was shown in Fig. 4.
 | ||
| Fig. 3 The structures of RAFT-PS-b-PMA (the linear copolymer matrix) and (RAFT-PS-co-PCMS-g-PMMA)-b-PMA (the graft copolymer matrix). | ||
2.4 Measurements
Molecular weights of macroinitiators (RAFT-PS and RAFT-PS-co-PCMS-g-PMMA) and copolymer matrixes were measured by gel permeation chromatography (GPC) on an Agilent1100 column using polystyrene standards as the calibration without any purification (flow rate: 0.6 mL min−1; temperature: 40 °C; solvent: THF; detector: RI). The contact angles of deionized water on the surface of copolymer matrixes in PDLC films were measured using a Kruss DSA100 goniometer system (Kruss GmbH, Germany). Each sample was tested five times, then the mean value of contact angle was recorded, and the surface energy of each sample was calculated.The response times of these PDLC films were measured by a LCD parameter tester (ZKY-LCDEO-2, from Chengdu Shiji Zhongke Instrument Co., Ltd.). The response of the sample was monitored by a digital storage oscilloscope. Rise time and decay time were defined as the time for the optical response (transmittance) to reach 90% and to drop 10% of the maximum transmittance, respectively.
3. Results and discussion
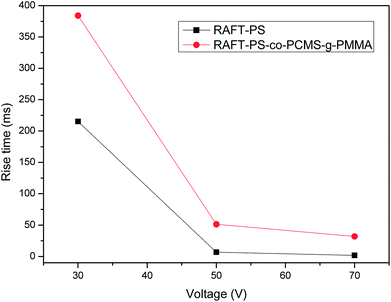
Fig. 5 showed the rise times of the samples with applied electric fields. It was evident that the rise time of every sample decreased with the increase of applied voltage from 30 V to 70 V. Under the same voltage, the PDLC with graft copolymer matrix exhibited slower rise time than linear one. It was concluded that the introduction of graft macroinitiator resulted in higher rise times. Meanwhile, decay times shown in Fig. 6 increased as the applied voltage increased from 30 V to 70 V. Under the same voltage, the PDLC with graft copolymer matrix exhibited faster decay time than linear sample. The higher the applied voltage was, the stronger external force would be, which meant rising quickly and decaying slowly. In conclusion, it was obvious that the response times of PDLCs with graft copolymer matrix were much different from linear samples. All PDLCs with graft copolymer matrixes exhibited slower rise times but faster decay times. | ||
| Fig. 6 Decay times dependence on the applied voltage for two samples with different macroinitiators. | ||
Why the introduction of graft macroinitiator could make so big difference in response times of PDLC films. We discussed carefully from the following aspects.
It was reported that the size of LC droplets,18 the anchoring energy on the boundary surface13 and the properties of polymer matrix27 were important factors to affect the anchoring force between LC droplets and polymer matrix, and therefore also the response times of PDLCs. On one hand, large LC droplets meant fast rise times but slow decay times.18 Based on the experimental results performed by us,27 graft copolymer matrix led to smaller LC droplets than linear one. Since the glass transition temperature (Tg) of branched chains (PMMA) was much higher than room temperature, these branched chains could prevent the LC droplets from gathering together during PIPS process, so smaller LC droplets were exhibited in graft macroinitiator dependent PDLCs. Munekazu Date has reported that the decrease of droplet size would result in higher anchoring force in the PDLC films because the ratio of the surface to volume was higher.29 Thus, PDLCs with graft copolymer matrixes showed higher rise times but lower decay times.
On the other hand, the variation of the molecular weights of copolymer matrix was also an important factor to affect the anchoring force. According to the literature, in RAFT polymerization, the calculated Mn could be predicted by eqn (1).20
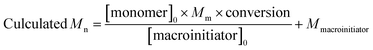 | (1) |
 | ||
| Fig. 8 Rise times of PDLCs dependence on the molecular weight of copolymer matrix and the applied voltage. | ||
 | ||
| Fig. 9 Decay times of PDLCs dependence on the molecular weight of copolymer matrix and the applied voltage. | ||
Regarding the anchoring energy on the boundary surface, in order to characterize it quantificationally, we calculated the surface energy by using Kruss DSA100 goniometer system, and the results were shown in Table 3. It was found that when the copolymer matrix was replaced from the linear to graft, however, the surface energy was decreased from 21.92 to 9.79 mN m−1. It was presumed that since the branched chains of graft copolymer matrix were not locomotive enough, this might restrict the movement of the polar end group to the boundary surface. So the anchoring energy on the boundary surface of the graft copolymer matrix was smaller than that of the linear one.
| Copolymer matrix | Contact angle (°) (water) | Surface energy (mN m−1) | ||
|---|---|---|---|---|
| L | R | M | ||
| Linear copolymer matrix | 101.8 | 101.8 | 101.8 | 21.92 |
| Graft copolymer matrix | 123.6 | 123.6 | 123.6 | 9.79 |
Based on the several points above, we proposed a possible explanation to this phenomenon. When copolymer matrix was replaced from the linear to the graft, the decrease of the radius of LC droplets and the increase of the molecular weight of copolymer matrix were the keys for the increase of the anchoring force, although the anchoring energy on the boundary surface was reduced in certain degree. Under the consequence of synthetic functioning of various factors, the anchoring force between LC droplets and the graft copolymer matrixes became much stronger and PDLCs with this polymer matrix exhibited higher rise time and lower decay time.
Furthermore, from another point of view, the change of driving voltage (the threshold voltage Vth and the saturation voltage Vsat) was in rather good agreement with the variation of anchoring force mentioned above. It was well known that there was a classical relationship between anchoring force and Vsat.30
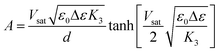 | (2) |
4. Conclusion
In conclusion, we discussed the effect of graft copolymer matrix on the response times of PDLCs by introducing branched chains on linear copolymer matrix. It was found that when the graft macroinitiator was employed to prepare copolymer matrix, PDLCs exhibited slow rise times but fast decay times. By introducing branched chains on linear copolymer matrix, on one hand, the gathering of LC droplets was prevented to a large extent, the LC droplets were very small; on the other hand, with the increase of molecular weight of copolymer matrix, the interaction between LC droplets and copolymer matrix became much stronger. These were the key factors which led to the variation of response times. This explanation corresponded well to the experimental results.Acknowledgements
This work was supported by National Natural Science Foundation of China (Grant no. 51173115), the Ministry of Education (the Foundation for Ph.D. training, Grant no. 20110181110030) of China and the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry, and Science (no. 20071108-18-12).Notes and references
- G. D. Filpo, F. P. Nicoletta and G. Chidichimo, Adv. Mater., 2005, 17, 1150 CrossRef.
- M. Macchione, G. D. Filpo, F. P. Nicoletta and G. Chidichimo, Chem. Mater., 2004, 16, 1400 CrossRef CAS.
- M. Mucha, Prog. Polym. Sci., 2003, 28, 837 CrossRef CAS.
- M. W. P. L. Baars, M. C. W. van Boxtel, C. W. M. Bastiaansen, D. J. Borer, S. H. M. Söntjens and E. W. Meijer, Adv. Mater., 2000, 12, 715 CrossRef CAS.
- D. A. Higgins, Adv. Mater., 2000, 12, 251 CrossRef CAS.
- J. H. Ryu, Y. H. Choi and K. D. Suh, Colloids Surf., A, 2006, 275, 126 CrossRef CAS.
- L. Bouteiller and L. P. Barny, Liq. Cryst., 1996, 21, 157 CrossRef CAS.
- G. H. Springer and D. A. Higgins, J. Am. Chem. Soc., 2000, 122, 6801 CrossRef CAS.
- J. W. Doane, N. A. Vaz, B. G. Wu and S. Zumer, Appl. Phys. Lett., 1986, 48, 269 CrossRef CAS.
- J. Erdmann, J. W. Doane, S. Zumer and G. Chidichimo, Proc. SPIE, 1989, 1080, 32 CrossRef CAS.
- B. G. Wu, Liq. Cryst., 2006, 33, 1315 CAS.
- P. S. Drzaic, Liq. Cryst., 2006, 33, 1286 CrossRef CAS.
- D. Cupelli, F. P. Nicoletta, G. D. Filpo, G. Chidichimo, A. Fazio, B. Gabriele and G. Salerno, Appl. Phys. Lett., 2004, 85, 3292 CrossRef CAS.
- P. Song, H. Cao, F. Wang, M. Ellahi and H. Yang, Liq. Cryst., 2012, 39, 903 CrossRef CAS.
- P. Song, H. Cao, F. Wang, F. Liu, J. Wang, M. Ellahi, F. Li and H. Yang, Liq. Cryst., 2012, 39, 1131 CrossRef CAS.
- Y. W. Jin, S. J. Im, J. H. Sung, C. H. Noh and D. S. Sakong, Liq. Cryst., 1995, 19, 755 CrossRef CAS.
- W.-J. Yang, T. Lan, S.-L. Xia, L.-P. Ma and Y.-H. Wang, Liq. Cryst., 2014, 41, 202 CrossRef CAS.
- J. L. West, K. Jewell, J. Francl, Y. M. Ji and J. R. Kelly, Proc. SPIE, 1992, 1665, 8 CrossRef CAS.
- L. Y. Ljungberg, Mater. Des., 2003, 24, 383 CrossRef CAS.
- J. He, B. Yan, S.-L. Wang, B.-Y. Yu, X.-A. Wang and Y.-H. Wang, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 4144 CrossRef CAS.
- J. He, B. Yan, B.-Y. Yu, S.-L. Wang, Y. Zeng and Y.-H. Wang, Eur. Polym. J., 2007, 43, 2745 CrossRef CAS.
- B. Yan, J. He, Y.-Q. Fang, X. Du, Q. Zhang, S.-L. Wang, C.-H. Pan and Y.-H. Wang, Eur. Polym. J., 2009, 45, 1936 CrossRef CAS.
- X. Du, B. Yan, J.-L. Yao, S. Chen, M.-K. Li and Y.-H. Wang, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 5557 CrossRef CAS.
- X. Du, B. Yan, M.-K. Li, S. Chen, J.-L. Yao and Y.-H. Wang, Polym. Int., 2011, 60, 971 CrossRef CAS.
- L.-S. Shao, Y.-L. Zhang, C.-H. Liu, J.-J. Li, A.-L. Qin and Y.-H. Wang, Liq. Cryst., 2012, 39, 1458 CrossRef CAS.
- T. Lan, W.-J. Yang, J. Peng, M.-K. Li and Y.-H. Wang, Polym. Eng. Sci. DOI:10.1002/pen.23857.
- T. Lan, W.-J. Yang, J. Peng, M.-K. Li and Y.-H. Wang, Polym. Int. DOI:10.1002/pi.4693.
- J. Skey and R. K. O'Reilly, Chem. Commun., 2008, 4183 RSC.
- M. Date, Y. Takeuchi and K. J. Kato, J. Phys. D: Appl. Phys., 1999, 32, 3164 CrossRef CAS.
- A. Sugimura and D. Ishino, Thin Solid Films, 2003, 433, 438 Search PubMed.
| This journal is © The Royal Society of Chemistry 2014 |