Aerobic synthesis of biocompatible copper nanoparticles: promising antibacterial agent and catalyst for nitroaromatic reduction and C–N cross coupling reaction†
Showmya Venkatakrishnana,
Ganapathy Veerappanb,
Elangovan Elamparuthia and
Anbazhagan Veerappan*a
aDepartment of Chemistry, School of Chemical and Biotechnology, SASTRA University, Thanjavur – 613 401, Tamil Nadu, India. E-mail: anbazhagan@scbt.sastra.edu.in; Fax: +91-4362-26120; Tel: +91-4362-264101-3689
bSKKU Advanced Institute of Nano Technology, Sungkyunkwan University, Suwon, South Korea
First published on 13th March 2014
Abstract
Herein, we report the synthesis of copper nanoparticles at ambient conditions using biopolymer, pectin, as a protecting agent and hydrazine as a reducing agent. The obtained nanoparticles catalyze the reduction of nitroaromatic compounds in aqueous solution and also catalyze the C–N cross coupling of amines with bromobenzene in good yields.
Metal nanoparticles find application in catalysis, biodiagnostics, drug delivery, nanomedicine, sensors, photonics and electronics.1 Among many metal nanoparticles, group IB metals such as silver, gold and copper have received considerable attention due to their attractive optical properties, arising from localized surface plasmon resonance in the visible and the near infrared region of the electromagnetic spectrum. In this list copper was less popular, mainly because the fabrication of chemically stable copper nanoparticles (CuNPs) was far more complicated, because they are prone to fast oxidation.2 In-spite of the difficulty, CuNPs have attracted considerable attention because they are much more economical than the silver and gold nanoparticles. Therefore, several methods have been developed for the preparation of copper nanoparticles, including chemical reduction, thermal reduction, pulsed sonoelectrochemical reduction, radiation methods, metal vapour synthesis, vacuum vapour deposition, microemulsion techniques and laser ablation.3 Most of these methods are either required sophisticated instrument or required biological hazardous chemicals and also required oxygen free environment to synthesize CuNPs. There are very few reports exist on CuNPs synthesis without inert atmosphere.4 Hence, a more robust, cost-effective and eco-friendly approach is still required to synthesize stable CuNPs.
Of late, green chemistry methods utilizing the sources such as microbes, plant extract and biopolymer have received significant attention in the synthesis of inorganic nanoparticles.5 However, most of the green chemistry approaches of nanoparticles synthesis have stopped at the level of synthesis and basic characterization. In order to effectively utilize the nanoparticles synthesized by green chemistry method, it is imperative to develop its application.
In this paper, we describe here the first preparation of pectin stabilized CuNPs at ambient conditions and report on their excellent catalytic activity for the reduction of various nitroaromatic compounds. We have also demonstrated for the first time that pectin protected CuNPs has an ability to catalyze C–N cross coupling of amines with bromobenzene. We have used pectin, a polysaccharide isolated from citrus peel, consisting of a linear backbone of (1–4) linked α-D-galacturonic acid residue with varying degree of methylesterified carboxyl groups, as a templating agent.6 It is widely used as gelling, thickening and stabilizing agents in the food industry. The choice of pectin was made because of its benign nature and the presence of oxygen rich functional group and acid group allows CuNPs to be very stable.
The preparation of CuNPs using biopolymer, pectin as a capping agent and hydrazine as a reducing agent was illustrated in Fig. 1A. In a typical experiment, 50 mM of CuCl2 was mixed with 50 mL aqueous suspension of pectin (1 mg mL−1) through vigorous stirring. The greenish blue gel acidic solution brought to basic by adding 200 μL of concentrated ammonia solution. The colour changes from greenish blue to blue due to the formation of copper–ammonia complex. After 5 minutes of gentle mixing, 200 μL of hydrazine hydrate was added as a mild reducing agent. After stirring the solution for 5 minutes, the container was left undisturbed in open air for 3 hours. The formation of copper nanoparticles was easily noticeable due to the change in the color of the solution to reddish brown – the characteristic color of the copper nanoparticles (Fig. 1A).
The UV-visible spectra of the CuNPs solution exhibit a characteristic surface plasmon resonance peak at 580 nm (Fig. 2A). Time dependent absorption studies of CuNPs showed that the NPs formation was accompanied by three stages (Fig. 2B). Stage I, initiation process, which occurs between 0 to 65 minutes. The conversions of copper complex to CuNPs (Stage II) occur between 65 minutes to 120 minutes. After 120 minutes, the absorption intensity does not change, indicating that the NPs formation was completed (Stage III). Analyzing the time dependent formation of NPs yielded the half time (95.06 minutes), the time required to convert half of the copper complex to CuNPs. The dispersion of the CuNPs in water was very clear and stable for more than 30 days without settling down. The initial reddish brown color of CuNPs remains stable, even exposing the CuNPs to open air for 10 days. The stability of CuNPs was also monitored by measuring their intensity at 580 nm over a period of 30 days and no significant change in the intensity was observed. The synthesized CuNPs examined by XRD displays high crystallinity. The thin film of CuNPs showed diffraction peak at 43.70°, 50.78° and 74.45°, corresponding to the crystal facets of (1 1 1), (2 0 0) and (2 2 0), respectively (Fig. 1B) and compared with the standard diffraction pattern of metallic copper (JCPDS card no. 4-0836). The pattern was very clean, with no indication of impurities such as copper oxides (CuO, Cu2O). The TEM images, as shown in Fig. 1C, suggested that the CuNPs are spherical in nature with size is about 5 nm.
 | ||
| Fig. 2 Formation of pectin stabilized CuNPs (A) time dependent UV studies of CuNPs formation, (B) time dependent changes in the absorption maximum at 580 nm. | ||
The catalytic activity of the synthesized CuNPs was measured by sodium borohydride (NaBH4) mediated reduction of p-nitrophenol (p-NP) to p-aminophenol (p-AP). The chosen reaction was easy to follow by UV-Vis absorption spectroscopy, therefore, this reaction have been used widely to evaluate the catalytic activity of various metallic NPs including gold, silver, nickel, palladium and platinum.7 Addition of NaBH4 to the aqueous solution of p-NP was accompanied by a colour change from light yellow to yellowish-green (Fig. 3A). The absorption maximum of p-NP shifts from 317 nm to 400 nm (Fig. 3B), suggested the formation of p-nitrophenolate anion. The addition of CuNPs was accompanied by color change from yellowish-green to colorless suggested the reduction of p-NP to p-AP (Fig. 3A). The absorption maximum at 400 nm in presence of CuNPs decreases with time and a new peak due to the formation p-AP appears at 298 nm (Fig. 3B). As shown in Fig. 3C, the concentration of p-NP at 400 nm remained unaltered in presence of NaBH4, indicating that the NaBH4 does not reduce p-NP in the absence of CuNPs.
To determine the reaction rate, we monitored time dependent changes in the absorption peak of p-NP at 400 nm in the presence of CuNPs. Before the catalytic reduction of p-NP, an induction time up to 120 s was observed (Fig. 3C). This induction time is typical for a heterogeneous catalytic process and commonly related to activation or restructuring of the metal surface by nitrophenol before the reaction could start.8 After an induction time t0, the reaction starts and completed within 250 s. In this catalytic reaction, the concentration of NaBH4 was much higher than that of p-NP, thus, the reduction kinetics can be described as pseudo first-order with respect to p-NP alone. Fig. 3C show the plots of ln(Ct/C0) versus reaction time for the reduction of p-NP, where Ct and C0 are the concentration of p-NP at time t and 0, respectively. The linear correlation between ln(Ct/C0) and the reaction time indicate that the reduction reaction follows pseudo first order kinetics. The rate constant, k (1.08 × 10−2 s−1) was obtained directly from the slope of the linear part of the kinetic trace.
The scope of the above described procedure has been examined with various nitroaromatic compounds (Fig. S1–S4†) and the results are summarized in Table 1. As noted from Table 1, the nitro group present at para position undergoes faster reduction with lesser induction time. When the nitro group is in ortho or meta position, the rate of reduction decreases considerably with higher induction time. This suggest that the activation or restructuring of the metal surface by the ortho or meta substituted nitro compound is delayed because of the steric hindrance and yielded lesser rate (Table 1). However, irrespective of the substituted position, all the nitro compounds are converted efficiently to their corresponding amino compounds in less than 5 minutes.
| Substrate | t0 (s) | (k) (s−1) |
|---|---|---|
| p-Nitrophenol | 120.7 | 1.08 × 10−2 |
| p-Nitrobenzamine | 79.8 | 1.32 × 10−2 |
| m-Nitrobenzamine | 160.2 | 0.21 × 10−2 |
| o-Nitrobenzamine | 169.8 | 0.68 × 10−2 |
| p-Methyl-o-nitrobenzamine | 139.8 | 0.75 × 10−2 |
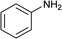
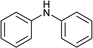
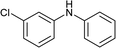
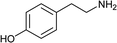
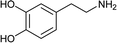
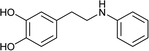
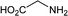
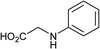
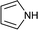
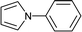
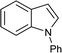
To further expand the scope of pectin stabilized CuNPs, we next explored the C–N cross coupling of amines with aryl halide. The formation of C–N bonds by cross coupling reaction represents a powerful method to prepare biological and pharmaceutical compounds.9 The reaction of aniline with bromobenzene was first studied as standard substrate with CuNPs act as a catalyst. The reaction occurred to produce diphenylamine in 85% yield when it was stirred at 110 °C in the presence of 2 mol% of CuNPs and 1 mol% of NaOH in DMSO under aerobic conditions. The success of diphenylamine formation encouraged us to extend the application of CuNPs to synthesis various C–N cross coupling product with aryl amines, alkyl amine and N-heterocyclic amines. 3-Chloro aniline, tyramine, dopamine, glycine, pyrrole and indole underwent reaction with bromobenzene to afford desired product in moderate to good yield. The obtained products was purified by flash chromatography and characterized by thin layer chromatography and 1H-NMR (details in ESI†). The results of these experiments are summarized in Table 2. The reusability of the catalyst was examined by reacting aniline with bromobenzene. After completion of the reaction, the catalyst was recovered by centrifugation at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm and reused for the fresh reaction of aniline with bromobenzene and observed no loss of activity even after 3 cycles.
000 rpm and reused for the fresh reaction of aniline with bromobenzene and observed no loss of activity even after 3 cycles.
The gradual increases in the identification of new multi-drug resistant bacteria strain have forced the research community to discover new antibacterial agent. Copper and its complex have been well known for the bactericidal effect.10 In order to exploit the pectin stabilized CuNPs against pathogenic bacteria we tested the bactericidal effect of CuNPs by disk diffusion method against Gram positive and Gram negative bacteria (Fig. S12 and S13†). As shown in Table 3, the diameter of inhibition zones around the disk containing CuNPs varies for different bacterial species. This difference in zone of inhibition among the bacterial species can be attributed to different cell wall composition. It is clear from Table 3 that the zone of inhibition of the CuNPs was comparable to the standard antibiotic, ofloxacin and kanamycin. The zone of inhibition in the present study was comparatively higher than the chitosan stabilized CuNPs and alginate stabilized CuNPs.11 While considering the mucoadhesive nature of pectin, the reported antibacterial activity of the newly synthesized pectin stabilized CuNPs is promising and required detail investigation for future application.
| Bacteria | Zone of inhibition (mm) | ||
|---|---|---|---|
| CuNPs | Ofloxacin | Kanamycin | |
| Bacillus thuringiensis | 16 | 16 | 14 |
| Staphylococcus aureus | 19 | 19 | 16 |
| Klebsiella pneumonia | 17 | 19 | 18 |
| Escherichia coli | 17 | 15 | 11 |
| Salmonella typhi | 12 | 16 | 12 |
| Pseudomonas aeruginosa | 16 | 14 | 17 |
| Shigella flexneri | 16 | 15 | 11 |
| Proteus mirabilis | 26 | 20 | 20 |
Conclusions
In conclusion, we presented a facile route to synthesize copper nanoparticles at room temperature. The materials used in this synthesis are biocompatible and the obtained product is air stable. The synthesized CuNPs catalyze the reduction of nitroaromatic compounds in aqueous solution and also catalyze the C–N cross coupling of amines with bromobenzene in good yields. They also display promising antibacterial activity, as good as ofloxacin and kanamycin, towards the tested bacteria. Further investigation on the catalytic property and antibacterial activity will enable the use of this nanomaterial in the field of nanomedicine and catalysis.Acknowledgements
The Department of Science and Technology, Government of India is acknowledged for financial support (SB/FT/LS-217/2012). We thank Central Research Facility, SASTRA University for providing the necessary infrastructure.Notes and references
- (a) P. K. Jain, X. Huang, I. H. El-Sayed and M. A. El-Sayed, Acc. Chem. Res., 2008, 411, 1578 CrossRef PubMed; (b) R. A. Sperling, P. Rivera-gil, F. Zhang, M. Zanella and W. J. Parak, Chem. Soc. Rev., 2008, 37, 1896 RSC; (c) B. C. Ranu, K. Chattopadhyay, L. Adak, A. Saha, S. Bhadra, R. Dey and D. Saha, Pure Appl. Chem., 2009, 81, 2337 CrossRef CAS.
- K. K. R. Datta, C. Kulkarni and M. Eswaramoorthy, Chem. Commun., 2010, 46, 616 RSC.
- (a) D. Kim, S. Jeong and J. Moon, Nanotechnology, 2006, 17, 4019 CrossRef CAS PubMed; (b) J. Hambrock, R. Becker, A. Birkner, J. Weiß and R. A. Fischer, Chem. Commun., 2002, 68 RSC; (c) I. Haas, S. Shanmugam and A. Gedanken, J. Phys. Chem. B, 2006, 110, 16947 CrossRef CAS PubMed; (d) A. A. Ponce and K. J. Klabunde, J. Mol. Catal. A: Chem., 2005, 225, 1 CrossRef CAS.
- (a) A. K. Chatterjee, R. K. Sarkar, A. P. Chattopadhyay, P. Aich, R. Chakraborty and T. Basu, Nanotechnology, 2012, 23, 85103 CrossRef PubMed; (b) M. Vaseem, K. M. Lee, D. Y. Kim and Y.-B. Hahn, Mater. Chem. Phys., 2011, 125, 334 CrossRef CAS; (c) Y. J. Lee, K. J. Lee, N. E. Stott and D. Kim, Nanotechnology, 2008, 19, 415604 CrossRef PubMed.
- (a) L. Sintubin, W. Verstraete and N. Boon, Biotechnol. Bioeng., 2012, 109, 2422 CrossRef CAS PubMed; (b) S. Iravani, Green Chem., 2011, 13, 2638 RSC.
- (a) H. V. Scheller, J. K. Jensen, S. O. Sørensen, J. Harholt and N. Geshi, Plant Physiol., 2007, 129, 283 CrossRef CAS; (b) B. L. Ridley, M. A. O'Neill and D. Mohnen, Phytochemistry, 2001, 57, 929 CrossRef CAS PubMed.
- (a) B. Liu, D. W. Zhang, J. C. Wang, C. Chen and X. L. Yang, J. Phys. Chem. C, 2013, 117, 6363 CrossRef CAS; (b) J. M. Zhang, D. H. Han, H. J. Zhang, Y. Zhao and D. L. Ma, Chem. Commun., 2012, 48, 11510 RSC; (c) Z. M. Zhu, X. H. Guo, S. Wu, R. Zhang, J. Wang and L. Li, Ind. Eng. Chem. Res., 2011, 50, 13848 CrossRef CAS; (d) X. Zhang and Z. H. Su, Adv. Mater., 2012, 24, 4574 CrossRef CAS PubMed.
- P. Hervés, M. Pérez-Lorenzo, L. M. Liz-Marzán, J. Dzubiella, Y. Lu and M. Ballauff, Chem. Soc. Rev., 2012, 41, 5577 RSC.
- (a) M. Negwar, in Organic-Chemical Drugs and their Synonyms: (An International Survey), Akademie, Berlin, Germany, 1994, 7th edn Search PubMed; (b) J. B. Buckingham, in Dictionary of Natural Products, Chapman and Hall, London, 1994, vol. 1 Search PubMed.
- (a) B. Jia, Y. Mei, L. Cheng, J. Zhou and L. Zhang, ACS Appl. Mater. Interfaces, 2012, 4, 2897 CrossRef CAS PubMed; (b) F. Gao, H. Pang, S. Xu and Q. Lu, Chem. Commun., 2009, 3571 RSC; (c) G. Borkow and J. Gabbay, FASEB J., 2004, 18, 1728 CAS.
- (a) M. S. Usman, M. E. El Zowalaty, K. Shameli, N. Zainuddin, M. Salama and N. A. Ibrahim, Int. J. Nanomed., 2013, 8, 4467 Search PubMed; (b) J. Díaz-Visurraga, C. Daza, C. Pozo, A. Becerra, C. von Plessing and A. García, Int. J. Nanomed., 2012, 7, 3597–3612 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Description of experimental procedure. UV-Vis spectra, kinetics data, 1H-NMR spectra and antimicrobial activity plates. See DOI: 10.1039/c4ra01126k |
| This journal is © The Royal Society of Chemistry 2014 |