Tuning the electrochemical performances of anthraquinone organic cathode materials for Li-ion batteries through the sulfonic sodium functional group†
Wang Wana,
Hungsui Leeb,
Xiqian Yu*b,
Chao Wanga,
Kyung-Wan Namc,
Xiao-Qing Yang*b and
Henghui Zhou*a
aCollege of Chemistry and Molecular Engineering, Peking University, Beijing, P. R. China. E-mail: hhzhou@pku.edu.cn; Fax: +86-10-62754680; Tel: +86-10-62754680
bBrookhaven National Laboratory, Upton, New York 11973, USA. E-mail: xyu@bnl.gov; xyang@bnl.gov; Tel: +1-631-3443663
cDepartment of Energy and Materials Engineering, Dongguk University Seoul, Seoul 100-715, Republic of Korea
First published on 7th April 2014
Abstract
The effects on electrochemical performance of C14H8O2 organic cathode materials with and without SO3Na– functional groups for lithium ion batteries were investigated. The Na2C14H6O8S2 with two SO3Na– shows the best cycle performance and highest lithium storage voltage, while an outstanding rate performance is also achieved after combination with graphene paper.
Based on the low cost, environmentally friendly nature, and great varieties of chemical compounds in a wide potential range, developing organic compounds for electrode materials has attracted a lot of attention in rechargeable battery research. A large number of electroactive organic compounds have been studied.1–11 Among them, quinone based compounds are especially interesting. It has been reported that quinone can be used as a cathode material for lithium ion batteries,9,12–17 with a suitable lithium storage potential in a range between 2 to 3 V vs. Li/Li+. However, like most other organic electrode materials,8,18,19 quinone also suffers from poor cycle performance, which is mainly due to its high solubility in electrolytes.16
A lot of effort has been made to resolve the dissolution issue of the organic electrode materials. Polymerization is one of the most effective approaches being widely used and reported in the literature. Song et al. synthesized a poly(anthraquinone sulfide) that showed excellent reversibility and cyclability.20 Wei et al. reported a polyimide/CNT composite showing a good cycle stability of up to 93% of the initial capacity after 300 cycles.8 Grafting the organic molecules on the insoluble substrates is another effective approach to suppress the dissolution of the organic materials. Genorio et al. immobilized quinone derivative materials by grafting them onto SiO2 nanoparticles and carbon black. Relying on the covalent grafting and electrochemical grafting, the quinone derivatives are quite stable during the entire cycling process.3,16 This method improved the cycle stability significantly but sacrificed a large amount of the specific capacity of the electrode materials. Recently, Zeng et al. reported a new anthraquinone derivate, Li2(C14H6O4), which can greatly inhibit the dissolution of the anthraquinone in electrolyte, resulting in good cycle performance.21 However, although the dissolution of anthraquinone was reduced by adding a lithium functional group (LiO–), the average lithium storage voltage was lowered, which is undesirable for cathode application.
Certain redox active centers of organic molecules (and also polymers) offer various combinations of atomic arrangements and functional group substitutions, which may provide capabilities for fine tuning their properties to the desirable performance.3,19,22–25 Therefore, introducing functional groups to the quinone molecule will not only open a new approach to solve the dissolution problems of organic cathode materials but also provide valuable guidance for tuning other properties such as specific capacity and lithium storage potential. In this paper, we reported a series of sodium functional groups (SO3Na–) modified anthraquinone (C14H8O2, AQ) compounds and studied the effects of these functional groups on the electrochemical properties of these compounds as cathode materials for Li-ion batteries. Since the specific capacity will decrease with an increasing number of the SO3Na– function groups per anthraquinone, the highest number of this functional group is limited to two and only anthraquinone-1-sulfonic acid sodium (NaC14H7O5S, AQS) and anthraquinone-1,5-disulfonic acid sodium (Na2C14H6O8S2, AQDS) salts were studied in comparison with no modified AQ in this work. It will be shown later in this paper that the interionic and/or intermolecular forces between sulfonic groups can suppress the dissolution of the modified anthraquinone compounds in electrolyte, resulting in a significant improvement in the cycle performance. In addition, a higher lithium storage voltage can be achieved through sulfonic modification, attributed to the electron withdrawing function of the sulfonic group.
Thermal stability is one important factor of the electrode materials that may influence their further modification.26 Therefore, the thermal stability of these three compounds was evaluated first by thermogravimetric (TG) analysis under a nitrogen atmosphere. It can be seen in Fig. S1† that the onset decomposition temperatures of AQ, AQS and AQDS (with 0, 1 and 2 SO3Na– groups) are around 250, 440 and 520 °C, respectively, indicating the increased thermal stability of the anthraquinone compounds with added SO3Na– functional groups, and AQDS shows the best thermal stability.
The investigated anthraquinone compounds are well crystallized as can be proved by the XRD patterns shown in Fig. S2.† SEM images were used to examine the morphology of these three compounds, and the results are shown in the inset images in Fig. 1b(A–C). It can be seen that the morphology of these three compounds is almost the same with particle size distribution at the micrometer level. Therefore, the electrochemical performance of these three compounds is not influenced much by the morphological differences of these samples. Fig. 1a plots the charge–discharge profiles of these three compounds recorded at a current density of 0.1 C-rate in the voltage range of 1.5–4 V. It is interesting to note that the Li storage voltage of these compounds is strongly affected by the sulfonic sodium functional group. The AQDS with two sulfonic sodium groups at the 1 and 5 sites of the AQ shows the highest lithium storage voltage at around 2.4 V, while the unmodified AQ shows the lowest average storage voltage at around 2.1 V. AQS has an average lithium storage voltage at around 2.25 V, which is between that of AQDS and AQ, indicating that after adding the SO3Na– functional group, the lithium storage voltage has been improved, which can be attributed to the electron withdrawing effect of the SO3Na– functional group. The electron withdrawing effect will reduce the electron density of carbon atoms nearby. Owing to the conjugated effect on the ring, there is also a strong effect on the carbonyl groups, whereby the electron density of the carbonyl groups is reduced while the oxidizability is enhanced. As a result, the redox potential has been improved.27 The cyclic voltammetry (CV) curves of AQS and AQDS, which are given in Fig. S3,† show two pairs of redox peaks that are similar to the result reported by Zeng et al.,21 indicating a two-step lithium storage reaction mechanism.
The cycle performance of these three compounds was investigated under 0.1 C-rate charge–discharge (Fig. 1b). The theoretical capacities of these three materials are provided in Table S1.† It is obvious that after modifying anthraquinone with a SO3Na– functional group, the cycle performance is significantly improved. The AQDS shows the best cycle performance. No obvious capacity fading can be observed during cycling, and a reversible specific capacity of 120 mA h g−1 can be retained after 100 cycles. For AQ with no SO3Na– functional group, although it shows the highest initial discharge capacity of around 270 mA h g−1, continued severe capacity fading is observed with subsequent cycling. A reversible capacity of only 40 mA h g−1 can be generated after 100 cycles. AQS with one SO3Na– functional group shows a higher specific capacity than AQDS at the first cycle, which is consistent with the trend shown in Table S1,† and it shows a cycle performance poorer than AQDS but better than AQ. We can also see that the coulombic efficiency of AQ is the lowest, ca. 80%, which is mainly due to the dissolution of AQ during the charge and discharge process. The coulombic efficiencies of the AQS and AQDS are around 100%.
The improved cycle performance achieved by adding a SO3Na– functional group can be attributed to the decreased compound dissolution in electrolyte, as shown by the ultraviolet (UV) spectroscopy analysis (Fig. S4†). When collecting the UV spectra, AQ, AQS and AQDS were dissolved in DMC solvent and the solutions were examined by UV spectroscopy individually. It can be seen that AQ/DMC solution shows a large absorption peak at around 350 cm−1, indicating a large amount of AQ can be dissolved in DMC solvent. After adding one SO3Na– functional group to form AQS, its solubility is greatly reduced. The AQDS with two SO3Na– functional groups shows the lowest solubility, as evidenced by the UV spectrum where the absorption peak assigned to AQDS is very weak.
The morphological changes of the electrodes after cycling were further examined by SEM to see the dissolution effects of these three compounds. Fig. 2 shows the SEM images of the fresh electrodes of AQ, AQS, and AQDS, and the electrodes before and after cycling at a current rate of 0.1 C. Fresh electrodes were examined directly, and all the other electrodes samples after cycling are collected by disassembling the coin cells which have kept for the same time of about 10 days, including the cycling time. It can be seen from Fig. 2a–c that the compounds in fresh electrodes are distributed uniformly in the electrodes with a micrometer-scale particle size. It is obvious from Fig. 2d that the morphology of the AQ electrode has changed a lot after placement in the coin cells for 10 days. The original blocks disappeared and some dendrite like crystals formed, indicating that AQ has dissolved in the electrolyte. The morphology of AQS electrodes after 10 days' storage in cells shows a few changes (Fig. 2e). Some particles become smaller, which is also due to the dissolution of AQS in electrolyte. However, the morphology of AQDS electrodes with two SO3Na– groups remains almost the same as the fresh electrode after holding in the cell for 10 days (Fig. 2f), indicating that the dissolution of AQDS compound in electrolyte is much less than AQS and AQ. This can also be proved by the morphology change of electrodes with cycling shown in Fig. 2g–l. It is obvious that after 5 cycles of AQ electrode, there are some holes appearing on the electrode and the AQ particles become smaller and fewer, indicating that most of the AQ particles are dissolved in the electrolyte. After 10 cycles, nearly no AQ particles can be observed. We can conclude that the dissolution speed can be quickened by the charge and discharge processes in coin cells. The dissolution phenomena of the AQS electrode after cycling has been improved very much by AQ, but the morphology of AQDS electrodes stays nearly the same even after cycling for 10 times, further indicating that the AQDS compound has the lowest solubility in electrolyte. It is clear that after adding a SO3Na– functional group, the dissolution phenomena is partially suppressed, and AQDS shows the best stability in electrolyte.
 | ||
| Fig. 2 SEM of AQ, AQS, and AQDS electrodes: fresh electrode (a–c); before cycling (d–f); after 5 cycles (g–f); and after 10 cycles (j–l), respectively. | ||
It has been reported that the lithium storage mechanism (schematically shown in Fig. S5†) of AQ is a transition between anthraquinone and anthranol salt.5,15 Whether it remains the same after SO3Na– modification was further studied through Fourier transformed infrared (FTIR) spectroscopy analysis. Fig. 3 shows the FTIR spectra of the half-discharged (red), fully discharged (green) and recharged (blue) AQDS collected along with the pristine AQDS (black). It can be concluded that the reaction center is still on the carbonyl group, as it can be seen that the peak pertaining to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O vibration (1695 cm−1) disappeared while the C–O vibration peaks (1020 cm−1 and 1105 cm−1) appeared upon discharging. The peaks centered at 1215 cm−1 and 1145 cm−1 are associated with the vibration of the sulfonic group. These peaks only show a slight position shift, which may be caused by the change in the molecular structure from anthraquinone to anthranol after discharge. This indicates the SO3Na– functional groups do not participate in the reaction. Therefore, it can be concluded that the higher the number of SO3Na– functional groups linked on AQ, the smaller the specific capacity that will be delivered. The lithium storage mechanism of the AQDS compound during charge and discharge can be described in the following equation:
O vibration (1695 cm−1) disappeared while the C–O vibration peaks (1020 cm−1 and 1105 cm−1) appeared upon discharging. The peaks centered at 1215 cm−1 and 1145 cm−1 are associated with the vibration of the sulfonic group. These peaks only show a slight position shift, which may be caused by the change in the molecular structure from anthraquinone to anthranol after discharge. This indicates the SO3Na– functional groups do not participate in the reaction. Therefore, it can be concluded that the higher the number of SO3Na– functional groups linked on AQ, the smaller the specific capacity that will be delivered. The lithium storage mechanism of the AQDS compound during charge and discharge can be described in the following equation:
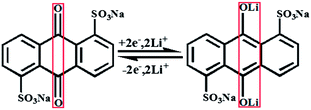 | (1) |
 | ||
| Fig. 3 FTIR spectra of AQDS compound at different charge and discharge stages as marked on the charge–discharge curve. | ||
This reaction is highly reversible, which can be proved by the similar FTIR features of the pristine and recharged AQDS shown in Fig. 3.
Since the AQDS exhibits excellent cycle performance, the rate capability was also examined under various C-rates at 0.2, 0.5, 1, 2, 3, 5 and 10 C. The rate performance of the AQDS is not too good with specific capacities of about 130, 110 and 90 mA h g−1 at 0.2 C, 0.5 C, and 1 C, respectively (as shown in Fig. 4). When the rate increased to 5 C, only 30 mA h g−1 reversible capacity can be obtained. The AQDS shows nearly no reversible capacity when the current density is up to 10 C. The poor rate capability is mainly cause by the poor electronic conductivity of this organic material, which is a common problem for most organic electrode materials.28 The reversible lithium storage behavior at 0.2 C can be recovered to 125 mA h g−1 after high rate cycling at 10 C, further indicating a good structural stability of AQDS.
 | ||
| Fig. 4 Rate performance of AQDS and AQDS/G paper electrodes. The inset figure shows the Nyquist plots of AQDS and AQDS/G paper electrodes. | ||
In order to improve the rate performance of AQDS compound, graphene as the most effective material to improve electronic conductivity is applied. An effective and facile approach has been explored to synthesis the AQDS and graphene paper composite (AQDS/G paper). It can be seen from the SEM images [Fig. 5(a) and (b)] that the AQDS particles were uniformly dispersed on the graphene paper. The as-prepared AQDS/G paper discs as shown in Fig. 5(c) were directly used as electrodes to evaluate the rate capability. The rate performances are provided in Fig. 4, and the corresponding charge–discharge curves at different rates are shown in Fig. S6.† It is obvious that AQDS/G paper has a much better rate performance in comparison with pure AQDS. The specific discharge capacities are around 130, 110, 100, 80, 70 and 60 mA h g−1 at 0.2, 0.5, 1, 2, 3, and 5 C rates, respectively. A reversible capacity of 50 mA h g−1 can be obtained even when cycled at a rate as high as 10 C. The corresponding charge–discharge curves at different C-rates can be seen in Fig. S6.†
Electrochemical impedance spectroscopy (EIS) experiments were performed to explain the superior rate performances of the AQDS/G paper (inset Fig. 4). The Nyquist plots of both AQDS and AQDS/G paper electrodes before cycling are inserted in Fig. 4. It is clear that the AQDS/G paper electrode exhibits a much lower charge transfer resistance than the pure AQDS electrode, which can be attributed to the enhanced conductivity of the AQDS/G paper. The equivalent circuit model was used to fit the Nyquist plots, and the fitted impedance parameters are listed in Fig. S7.† The pure AQDS electrode shows an Rct of 740 Ω, much larger than that of the AQDS/G paper electrode, which is only 438 Ω. This confirms that the incorporation of the graphene paper can greatly enhance the conductivity of AQDS and hence expedite the electron transport during the electrochemical lithium insertion/extraction reaction, leading to significantly improved electrochemical performances.
Conclusions
In summary, the effects of the SO3Na– functional groups on the electrochemistry performances of the anthraquinone compounds were studied. It was found that the SO3Na– is electrochemically inactive during the electrochemical process. Therefore, the specific capacity of the anthraquinone compounds decreases with an increasing number of the SO3Na– groups. On the other hand, the structural stability and the electrochemical cycle performance are significantly improved, due to the inorganic feature of the SO3Na– group. The anthraquinone with two SO3Na– functional groups (AQDS) achieves excellent cycle performance as well as moderate reversible capacity. In addition, the average lithium storage voltage increases with an increasing number of SO3Na– groups. The SO3Na– modification plays multi-functional roles both in solving the dissolution problem of the anthraquinone compounds in electrolytes and in tuning the lithium storage voltage. These results will provide valuable guidance in designing other organic electrode materials. Furthermore, an AQDS/graphene paper electrode was synthesized through a simple and efficient method, which can significantly improve the electronic conductivity of the electrodes and greatly benefit the rate capability. It will provide a facile approach to optimize the electrochemical performance of the organic electrode materials.Acknowledgements
We appreciate financial support from the A*Star Singapore-China Joint Research Program (no. 2012DFG52130). This work at BNL was supported by the U.S. Department of Energy, the Assistant Secretary for Energy Efficiency and Renewable Energy, Office of Vehicle Technologies under Contract Number DEAC02-98CH10886. The support provided by the China Scholarship Council (CSC) during a visit of Wang Wan to Brookhaven National Lab is acknowledged. The authors acknowledge technical support by the beamline scientist Dr Jianming Bai at X14A (NSLS, BNL).Notes and references
- X. Han, C. Chang, L. Yuan, T. Sun and J. Sun, Adv. Mater., 2007, 19, 1616–1621 CrossRef CAS.
- M. Armand, S. Grugeon, H. Vezin, S. Laruelle, P. Ribiere, P. Poizot and J. M. Tarascon, Nat. Mater., 2009, 8, 120–125 CrossRef CAS PubMed.
- B. Genorio, K. Pirnat, R. Cerc Korosec, R. Dominko and M. Gaberscek, Angew. Chem., Int. Ed., 2010, 49, 7222–7224 CrossRef CAS PubMed.
- P. Poizot and F. Dolhem, Energy Environ. Sci., 2011, 4, 2003–2019 CAS.
- Y. Liang, Z. Tao and J. Chen, Adv. Energy Mater., 2012, 2, 742–769 CrossRef CAS.
- S. Renault, D. Brandell, T. Gustafsson and K. Edstrom, Chem. Commun., 2013, 49, 1945–1947 RSC.
- L. Zhao, J. Zhao, Y. Hu, H. Li, Z. Zhou, M. Armand and L. Chen, Adv. Energy Mater., 2012, 2, 962–965 CrossRef CAS.
- Z. Wei, H. Wu, K. Wang, Y. Meng and K. Lu, J. Mater. Chem. A, 2013, 1, 6366–6372 Search PubMed.
- W. Xu, A. Read, P. K. Koech, D. Hu, C. Wang, J. Xiao, A. B. Padmaperuma, G. L. Graff, J. Liu and J. Zhang, J. Mater. Chem., 2012, 22, 4032–4039 RSC.
- Z. Song, H. Zhan and Y. Zhou, Angew. Chem., Int. Ed., 2010, 49, 8444–8448 CrossRef CAS PubMed.
- C. Luo, Y. Zhu, Y. Xu, Y. Liu, T. Gao, J. Wang and C. Wang, J. Power Sources, 2014, 250, 372–378 CrossRef CAS PubMed.
- Y. Liang, P. Zhang and J. Chen, Chem. Sci., 2013, 4, 1330–1337 RSC.
- Z. Lei, W. Weikun, W. Anbang, Y. Zhongbao, C. Shi and Y. Yusheng, J. Electrochem. Soc., 2011, 158, A991 CrossRef PubMed.
- H. Chen, M. Armand, M. Courty, M. Jiang, C. P. Grey, F. Dolhem, J. M. Tarascon and P. Poizot, J. Am. Chem. Soc., 2009, 131, 8984–8988 CrossRef CAS PubMed.
- L. Zhao, W. Wang, A. Wang, K. Yuan, S. Chen and Y. Yang, J. Power Sources, 2013, 233, 23–27 CrossRef CAS PubMed.
- K. Pirnat, R. Dominko, R. Cerc Korosec, G. Mali, B. Genorio and M. Gaberscek, J. Power Sources, 2012, 199, 308–314 CrossRef CAS PubMed.
- C. Luo, R. Huang, R. Kevorkyants, M. Pavanello, H. He and C. Wang, Nano Lett., 2014, 14, 1596–1602 CrossRef CAS PubMed.
- L. Zhan, Z. Song, N. Shan, J. Zhang, J. Tang, H. Zhan, Y. Zhou, Z. Li and C. Zhan, J. Power Sources, 2009, 193, 859–863 CrossRef CAS PubMed.
- T. Nokami, T. Matsuo, Y. Inatomi, N. Hojo, T. Tsukagoshi, H. Yoshizawa, A. Shimizu, H. Kuramoto, K. Komae, H. Tsuyama and J. Yoshida, J. Am. Chem. Soc., 2012, 134, 19694–19700 CrossRef CAS PubMed.
- Z. Song, H. Zhan and Y. Zhou, Chem. Commun., 2009, 448–450 RSC.
- R. Zeng, X. Li, Y. Qiu, W. Li, J. Yi, D. Lu, C. Tan and M. Xu, Electrochem. Commun., 2010, 12, 1253–1256 CrossRef CAS PubMed.
- J. Geng, J. P. Bonnet, S. Renault, F. Dolhem and P. Poizot, Energy Environ. Sci., 2010, 3, 1929–1933 CAS.
- D. J. Kim, S. H. Je, S. Sampath, J. W. Choi and A. Coskun, RSC Adv., 2012, 2, 7968–7970 RSC.
- S. E. Burkhardt, J. Bois, J. M. Tarascon, R. G. Hennig and H. D. Abruña, Chem. Mater., 2012, 25, 132–141 CrossRef.
- S. Wang, L. Wang, K. Zhang, Z. Zhu, Z. Tao and J. Chen, Nano Lett., 2013, 13, 4404–4409 CrossRef CAS PubMed.
- M. Armand and J. M. Tarascon, Nature, 2008, 451, 652–657 CrossRef CAS PubMed.
- L. F. Fieser and M. Fieser, J. Am. Chem. Soc., 1935, 57, 491–494 CrossRef CAS.
- W. Walker, S. Grugeon, O. Mentre, S. Laruelle, J. M. Tarascon and F. Wudl, J. Am. Chem. Soc., 2010, 132, 6517–6523 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details of making graphene oxide, table of the general properties of AQ, AQS and AQDS, XRD patterns, CV curves, UV spectra, charge–discharge curves and equivalent circuit model of the corresponding Nyquist plots. See DOI: 10.1039/c4ra01166j |
| This journal is © The Royal Society of Chemistry 2014 |


