Selective synthesis and comparative activity of olefinic isomers of 1,2-benzothiazine-1,1-dioxide carboxylates as aldose reductase inhibitors†
Shagufta Parveen,
Saghir Hussain,
Shaojuan Zhu,
Xiangyu Qin,
Xin Hao,
Shuzhen Zhang,
Jianglu Lu and
Changjin Zhu*
Department of Applied Chemistry, Beijing Institute of Technology, No. 5, Zhongguancun South Street, 100081 Beijing, China. E-mail: zcj@bit.edu.cn; Fax: +86 10 68918506; Tel: +86 10 68918506
First published on 21st March 2014
Abstract
α,β- and β,γ-unsaturated carboxylate isomers of 1,2-benzothiazine-1,1-dioxide were selectively synthesized via the Wittig olefination reaction under various temperature conditions. At 40 °C, α,β-unsaturated esters with high Z-stereoselectivity (83–87%) were formed, while β,γ-unsaturated esters formed preferentially with moderate to excellent regioselectivity at 100–120 °C (77–96%). The acid isomers were found to inhibit aldose reductase in order of activity β,γ-unsaturated > Z-α,β-unsaturated > E-α,β-unsaturated isomers. The β,γ-unsaturated isomer 7b, 2-[2-(4-bromo-2-fluorobenzyl)-1,1-dioxido-2H-1,2-benzothiazin-4(3H)-ylidene]acetic acid, exhibited the most potent inhibition activity, with an IC50 value of 0.057 μM. This was further supported by docking studies.
Introduction
The pathogenesis of diabetic complications such as retinopathy, neuropathy, cataracts, and stroke, is attributed to multiple biochemical pathways. Substantial evidence suggests a key role for the hyperglycaemia-initiated polyol pathway, advanced glycation end products, and oxidative stress in diabetic complications.1,2 Aldose reductase (ALR2, EC 1.1.1.21), which catalyses NADPH-dependent reduction of glucose to sorbitol, is the rate-determining enzyme of polyol pathway. It has been identified as a key target for therapeutic intervention to prevent and treat diabetic complications.3–5Therefore, aldose reductase inhibitors (ARIs) are becoming promising potential drugs. In the past few decades, a number of compounds have been developed as ARIs. Some selected ARIs are shown in Fig. 1.6–10
However, many failed to advance through the later stages of clinical trials or to lead to success on the market. This was owing to their poor efficacy or side effects, which were often associated with deficiency of ARI selectivity. That deficiency was measured by inhibitory activity against aldehyde reductase (EC 1.1.1.2, ALR1). Both ALR1 and ALR2 are members of the aldo-keto reductase (AKR) superfamily. They share a high number of sequences and structural homology, with the majority of differences appearing at the C-terminal end of the enzymes.11,12
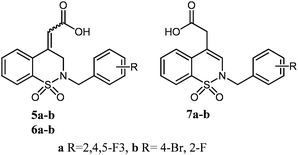
We have recently designed several series of potent ARIs based on the scaffolds of 1,2,4-benzothiazine-1,1-dioxide,8 pyrido[2,3-e][1,2,4]thiadiazine-1,1-dioxide9,13 and 1,2-benzothiazine-1,1-dioxide.14 The 1,2-benzothiazine-1,1-dioxide series, including C4-α,β-unsaturated and C4-acetic acids (Fig. 1), were prepared. Nevertheless, the stereochemistry of the α,β-double bond and the C4 carbon remained unclear. Therefore, it is of importance to achieve stereo-controlled synthesis of 1,2-benzothiazine-1,1-dioxide acids, and subsequently to demonstrate the effect of the orientation of the carboxylic acid group over the activity of aldose reductase inhibition.
The Wittig reaction15,16 proceeds in a stereoselective fashion for numerous substrates.17,18 It is demonstrative of a collection of versatile transformations in natural product synthesis19–21 and industrial processes.22–24 Previously, we reported the formation of α,β-unsaturated (exocyclic olefine) esters of 1,2-benzothiazine-1,1-dioxide.14 More recently, we have found the formation of only β,γ-unsaturated (endocyclic olefine) ester isomers at higher temperature. The present study focuses on the regio- and stereo-selective synthesis of 1,2-benzothiazine-1,1-dioxide ester isomers via Wittig olefination. Furthermore, it also deals with the behaviour of conformations of the carboxylic acid group on the inhibitory activity against aldose reductase.
Results and discussion
The starting materials 1a and b were prepared from commercially available sodium saccharin, which, on reacting with methyl bromoacetate under mild conditions, produces methyl [1,1-dioxido-3-oxo-1,2-benzothiazol-2(3H)-yl] acetate in excellent yield.25 Gabriele–Columan type ring expansion from a 5-membered isothiazol ring to a 6-membered thiazole ring yielded methyl 4-hydroxy-2H,1,2-benzothiazine-3-carboxylate 1,1-dioxide under an inert atmosphere.26,27 This resulted in the formation of 2H-1,2-benzothiazine-4(3H)-one when refluxed with concentrated hydrochloric acid. The resulting product provided N-substituted ketone 1a and b by alkylation with commercially available benzyl bromide in three steps.14,28 1a and b were allowed to react with the stabilized ylide of methyl 2-(triphenylphosphoranylidene) acetate to produce isomers of 1,2-benzothiazine 1,1-dioxide carboxylates (Scheme 1). The Wittig olefination was carried out at different temperatures (40–120 °C) in toluene under salt free conditions, and subsequently afforded Z, E, and endocyclic olefinic isomers with moderate to good stereo- and regio-selectivity. Wittig reaction of stabilized ylides (Ph3PCHR, R = EWG) normally gives olefins with high E selectivity under conditions of kinetic control.29 Anomalous Z selectivity depending on substrate and solvent is also reported.30–32The reaction of 1a and b 40 °C proceeded with high Z stereoselectivity (76%; 2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3a = 87
3a = 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13) and (89%; 2b
13) and (89%; 2b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3b = 83
3b = 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17), respectively (Fig. 2).
17), respectively (Fig. 2).
High Z selectivity can be explained by considering the [2 + 2]-cycloaddition mechanism.33 Two stable half chair conformations, A and B, are regularly suggested for the heterocyclic hexene ring, as shown in Fig. 3. When methyl 2-(triphenylphosphoranylidene) acetate approaches conformation A, oxaphosphetane transition state I is formed. The parallel orientation of the carbonyl and C–CO2Me dipoles and the high steric repulsion between the phenyl ring of benzothiazine and C–CO2Me cause instability. No such destabilizing factors are involved in the formation of II, as shown in Fig. 3. As a result, stereospecific decomposition of oxaphosphetane diastereomers leads to a high yield of the Z-alkene with phosphine oxide as the side product.
Isomerization of exocyclic to endocyclic olefins 4a and b was also observed with good to excellent regioselectivity at high temperature (Fig. 2). The products from the reaction of 1a at 100 °C were obtained in a yield of 88.9% (2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3a
3a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4a = 17
4a = 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6
6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 77). However, those from the reaction of 1b at 120 °C were obtained in a yield of 84.5% (2b
77). However, those from the reaction of 1b at 120 °C were obtained in a yield of 84.5% (2b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3b
3b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4b = 2
4b = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 96). These results are in good agreement with previous observations of Wittig olefination of monoketone and diketone with stabilized phosphorus ylide.34 These results indicate that the endocyclic olefins are thermodynamically stable.
96). These results are in good agreement with previous observations of Wittig olefination of monoketone and diketone with stabilized phosphorus ylide.34 These results indicate that the endocyclic olefins are thermodynamically stable.
However, the rapid fall in yield of 4a at 120 °C (Fig. 2) may result from the decomposition of the product at this temperature. A similar result was also observed in the case of 4b when temperature was higher than 120 °C.
The ester isomers afforded their corresponding exocyclic and endocyclic olefine acid isomers 5a and b, 6a and b, and 7a and b, respectively, by acid hydrolysis. However, base hydrolysis of all isomers gave only 7a and b.
Compound structure and stereochemistry
All of the synthesized compounds gave satisfactory spectroscopic data. The structures and stereochemistry of Z, E and endocyclic olefine isomers were determined. Firstly, the stereochemistry of geometrically pure isomers was determined. The differences in the chemical shifts of protons present at α, γ, and methylene protons of the substituted N-benzyl group are present. For instance, chemical shift (δ) values of a single proton at the α or γ position for 2a, 3a, and 4a are 6.76 ppm, 6.23 ppm, and 7.01 ppm, respectively.Furthermore, NMR-NOESY showed the NOE effect between the α-proton of 2a and b and the proton at the phenyl ring of the benzothiazine 1,1-dioxide framework. This indicated that the methyl ester group is on the opposite side to the phenyl ring of benzothiazine 1,1-dioxide, assigning the Z-configuration for 2a and b. The E-configuration for 3a and b was also assigned in the same way. In the case of 4a and b, NOESY correlations of the olefinic C3-proton of benzothiazine 1,1-dioxide framework were observed with two benzyl ring protons and two α-protons, respectively. However, no such correlation was observed between α-protons and any phenyl ring proton of the benzothiazine 1,1-dioxide framework. This allows an assignment of endocyclic non-conjugated structure for 4a and b (see ESI †). In addition, the structures and stereochemistry of compounds 2a and 4a were further confirmed by X-ray crystallography (Fig. 4).
 | ||
| Fig. 4 X-ray crystallography of 2a and 4a: H = white, C = gray, O = red, S = yellow, N = blue, F = cyan. | ||
Biological activity
The synthesized olefinic acid isomers of 1,2-benzothiazine 1,1-dioxide were tested for their inhibitory activity on ALR2 isolated from rat lenses. This is performed to identify the structure or stereochemistry crucial for optimal performance. A great difference in the activity between these isomers was observed, as shown in Table 1. The comparison of inhibitory ability in the a-series compounds showed that Z-isomer 5a was more active than E-isomer 6a, whereas the endocyclic olefine isomer 7a was the most active in this series (Table 1). Comparison of the b-series compounds gave the same results. In particular, the endocyclic olefine isomer 7b was found to be a potent ARI with an IC50 value of 0.057 μM.Consequently, olefinic isomers of 1,2-benzothiazine-1,1-dioxide carboxylic acid behaved differently with ranking order of endocyclic olefine isomers > Z-isomers > E-isomers. The endocyclic olefine isomers 7a and b showed the highest efficiency as compared to the other isomers. This was probably because of the flexible orientation of the C4-acetic acid moiety being made by the β,γ-double bond. This provided a more favourable orientation for the interaction between the acid head and the anion binding site of ALR2.
Molecular modeling
Molecular docking of the two series of compounds was performed on human ALR2 complexed with NADP+ and IDD594 (PDB code = 1US0). The docking results may explain the above mentioned stereostructure–activity relationship, as shown in Fig. 5. All three isomers of each series revealed significantly different patterns of binding interactions. In the case of a-series compounds, Z-isomer 5a formed three hydrogen bonding interactions to occupy the active site of the enzyme. The OH group of the carboxylate head formed H-bonds with Tyr48 (3.02 Å) and His110 (2.73 Å) in the anion binding pocket. The carbonyl oxygen atom also formed a hydrogen bond with the side chain of Cys298 (3.09 Å), as shown in (Fig. 5A). These three hydrogen bonding interactions might make the Z-isomer a stronger inhibitor as compared to the E-isomer 6a (Fig. 5B), since the carboxylate head of 6a formed only two hydrogen bond interactions with OH of Tyr48 (2.71 Å) and NE2 of His110 (2.32 Å). The endocyclic isomer 7a appeared to reside in a twisted conformation, held in the anion binding pocket by four hydrogen bonding interactions. The OH group of the carboxylic head formed two hydrogen bonds with NE2 of His110 (2.69 Å) and NE1 of Trp111 (3.44 Å), respectively, whereas the carbonyl oxygen has a hydrogen bond to the OH group of Tyr48 (2.77 Å). One of the oxygens of the thionyl group twisted to form a hydrogen bond with NE1 of Trp111 (3.21 Å). The benzyl rings of all ligands formed a stacking interaction against the indole ring of Trp111 side chain. Therefore, it is well placed into the specificity pocket (Fig. 5C).In case of the b-series of compounds, 5b and 6b showed similar patterns of interactions as those of 5a and 6a, respectively. However, the endocyclic olefine isomer 7b (Fig. 5D) behaved differently. Herein, the OH of the carboxylate head formed two hydrogen bonds. One hydrogen bond with OH of Tyr48 (2.97 Å) and another with NE2 of the imidazole ring of His110 (2.91 Å). A hydrogen bond is formed between a thionyl oxygen and NE1 of the indole ring of Trp111 (2.99 Å). An extra strong hydrogen bond (2.39 Å) between C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of the carboxylate head and the side chain of Cys298 is present. This bond may be responsible for its highest activity.
O of the carboxylate head and the side chain of Cys298 is present. This bond may be responsible for its highest activity.
Conclusions
Three olefinic carboxylate isomers of 1,2-benzothiazine-1,1-dioxide were synthesized with excellent stereo- and regio-selectivity in high yield under various temperature conditions. At 40 °C only α,β-unsaturated esters were formed with high Z-selectivity. A higher temperature of 100–120 °C resulted in β,γ-unsaturated esters being favoured, with moderate to excellent regioselectivity under base-free conditions. The stereochemistry was defined after successful separation and recrystallization of isomers. Comparative ALR2 inhibitory activity of these isomers demonstrated a key role of the orientation of the C4-carboxylic acid head of the compounds.Experimental
Melting points were recorded on a XT4A microscopic melting points apparatus and uncorrected. Thin layer chromatography (TLC) was performed on silica gel Merck 60F254. Nuclear Magnetic Resonance (NMR) spectra were recorded using a 400 MHz spectrometer (1H NMR at 400 MHz and 13C NMR at 100 MHz) in [d6]-DMSO using TMS as an internal reference. 1H NMR data are reported as follows: chemical shifts (δ, ppm), multiplicity (s = singlet, d = doublet, dd = doublet of doublets, t = triplet, q = quartet, m = multiplet, br = broad), integration, coupling constants (Hz). Data for 13C NMR are reported in terms of chemical shifts (δ, ppm). Individual resonances were assigned on the basis of their chemical shifts, signal intensities, multiplicity of resonances and coupling constants. MS was performed on Varian 500 MS ion trap mass by the ESI method, and HRMS (ESI-TOF) was performed on Agilent 6210 TOF. FTIR spectra was obtained from PerkinElmer Spectrum One FTIR spectrometer.General synthetic procedure for 2–4(a and b)
A solution of triphenylphosphine (26.2 g, 0.1 mol) in benzene (300 mL) was stirred for 15 minutes. This was followed by dropwise addition of a solution of methyl bromoacetate (1.5 eq.) in benzene (100 mL) over 1 h. The solid was filtered and washed with petroleum ether to provide a white solid phosphonium product. The crude product was dissolved in water (800 mL) and made alkaline by adding Et3N dropwise until the solution became basic enough. The reaction mixture was stirred for 1 h. The precipitates thus obtained were filtered and washed with water to provide the crude product, methyl 2-(triphenyl phosphoranylidene) acetate. The crude product was purified by recrystallization from methanol.A solution of an appropriate compound 1 (6 mmol) and methyl 2-(triphenylphosphoranylidene) acetate (9 mmol) in toluene (30 mL) was stirred at 40 °C to 120 °C until the reaction was complete. After evaporation of the solvent, the residue was purified by silica gel column chromatography (ethylacetate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) petroleum ether; 1
petroleum ether; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15–1
15–1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) to acquire the pure product.
20) to acquire the pure product.
General method for acid 5–7(a and b)
A mixture of the appropriate ester 2–4 (0.5 mmol), 1,4-dioxane (5 mL) and 10 M HCl (8 mL) was refluxed in the temperature range 60–80 °C. After completion of the reaction, ice cold water (5 mL) was added. The precipitate formed was then filtered and washed with cold water. The crude product was purified by flash chromatography and recrystallized from ethanol.Biological assay
ALR2 was prepared by following the method of Hayman and Kinoshita35 and Motta et al.36 The enzyme was extracted from freshly obtained rat lenses. The lenses were ground and homogenized in three volume of deionized water and centrifuged at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 30 min at 0–4 °C. The supernatant was skimmed off and precipitated with (NH4)2SO4. The precipitates were dissolved in 0.05 M NaCl solution and dialyzed overnight which was further used for inhibition assay. NADP, D,L-glyceraldehyde, and all other chemicals of reagent grade were purchase from Sigma-Aldrich. Epalrestat was prepared according to the reported method.
000 rpm for 30 min at 0–4 °C. The supernatant was skimmed off and precipitated with (NH4)2SO4. The precipitates were dissolved in 0.05 M NaCl solution and dialyzed overnight which was further used for inhibition assay. NADP, D,L-glyceraldehyde, and all other chemicals of reagent grade were purchase from Sigma-Aldrich. Epalrestat was prepared according to the reported method.
Enzyme inhibition activity was assayed on a Shimadzu UV-1800 UV spectrophotometer. This assay was carried out by measuring the decrease in absorption of NADPH at λ 340 nm, which accompanies the oxidation of NADPH catalyzed by ALR2. ALR2 activity was performed at 32 °C in a reaction mixture containing 0.25 mL of 0.10 mM NADPH, 0.25 mL of 0.1 M sodium phosphate buffer (pH 6.2), 0.1 mL enzyme extract, 0.15 mL deionized water, and 0.25 mL of 10 mM D,L-glyceraldehyde as the substrate, in a final volume of 1 mL. The reaction mixture, except for D,L-glyceraldehyde, was incubated at 32 °C for 10 min. The substrate was then added to start the reaction, which was monitored for 4 min.
The inhibitory activity of the newly synthesized compounds against ALR2 was assayed, adding 5 μL of the inhibitor. The inhibitory effect of the synthetic compounds was routinely estimated at a concentration of 10−4 M (the concentration refers to that of the compound in the reaction mixture).
The compounds found to be active were tested at additional concentrations between 10−4 and 10−8 M. Each dose–response curve was generated using at least four concentrations of compound with inhibitory activities between 20% and 80%, with three replicates at each concentration. The 95% confidence limits (95% CL) were calculated from t values for n − 2, where n is the total number of determinations.
Docking studies were performed using Molegro Virtual Docker, version 5.0. The crystal structure of human aldose reductase with bound inhibitor IDD594 (PDB code: 1US0) retrieved from the RCSB Protein Data Bank. All solvent molecules within the protein structure were removed for the docking procedure. Five binding cavities were detected to get the potential binding site in the protein. The cavity around the anion binding site (volume of ∼124 Å3) was chosen using the grid-based MolDock score (GRID) function with a grid resolution of 0.30 Å. The lowest energy ligand poses were chosen on the basis of the MolDock score and ReRank score. All structural parameters of ligands, such as bond orders, hybridization, explicit hydrogen atoms, and charges, were assigned when necessary in the Molegro Virtual Docker software.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant no. 21272025), the Research Fund for the Doctoral Program of Higher Education of China (grant no. 20111101110042), and the Science and Technology Commission of Beijing, China (grant no. Z131100004013003).References
- K. H. Gabbay, L. O. Merola and R. A. Field, Science, 1966, 151, 209–210 CAS.
- D. M. Nathan, N. Engl. J. Med., 1988, 318, 1315–1321 CrossRef PubMed.
- S. S. Chung and S. K. Chung, Curr. Med. Chem., 2003, 10, 1375–1387 CrossRef CAS.
- J. H. Kinoshita and C. Nishimura, Diabetes/Metab. Rev., 1988, 4, 323–337 CrossRef CAS.
- C. Yabe-Nishimura, Pharmacol. Rev., 1998, 50, 21–33 CAS.
- R. Kikkawa, I. Hatanaka, H. Yasuda, N. Kobayashi, Y. Shigeta, H. Terashima, T. Morimura and M. Tsuboshima, Diabetologia, 1983, 24, 290 CrossRef CAS.
- K. Sestanj, F. Bellini, S. Fung, N. Abraham, A. Treasurywala, L. Humber, N. Simard-Duquesme and D. Dvornik, J. Med. Chem., 1984, 27, 255 CrossRef CAS.
- X. Chen, C. J. Zhu, F. Guo, X. W. Qiu, Y. C. Yang, S. Z. Zhang, M. L. He, S. Parveen, C. J. Jing, Y. Li and B. Ma, J. Med. Chem., 2010, 153, 8330–8344 CrossRef PubMed.
- S. Zhang, X. Chen, S. Parveen, S. Hussain, Y. Yang, C. Jing and C. J. Zhu, ChemMedChem, 2013, 8, 603–613 CrossRef CAS PubMed.
- S. J. Ao, Y. Shingu, C. Kikuchi, Y. Notsu and I. Yamaguchi, Metab., Clin. Exp., 1991, 40, 77 CrossRef CAS.
- D. A. Carper, G. Wistow, C. Nishimura, C. Graham, K. Watanabe, Y. Fujii, H. Hayashi and O. Hayaishi, Exp. Eye Res., 1989, 49, 377–388 CrossRef CAS.
- O. A. Barski, K. H. Gabbay, C. E. Grimshaw and K. M. Bohren, Biochemistry, 1995, 34, 11264–11275 CrossRef CAS.
- Y. C. Yang, S. Z. Zhang, B. B. Wu, M. M. Ma, X. Chen, X. Y. Qin, M. L. He, S. Hussain, C. J. Jing, B. Ma and C. J. Zhu, ChemMedChem, 2012, 7, 823–835 CrossRef CAS PubMed.
- X. Chen, Y. C. Yang, B. Ma, S. Z. Zhang, M. L. He, D. Q. Gui, S. Hussain, C. J. Jing, C. J. Zhu and Q. Yu, Bioorg. Med. Chem., 2011, 19, 7262–7269 CrossRef CAS PubMed.
- G. Wittig and G. Geissler, Justus Liebigs Ann. Chem., 1953, 580, 44 CrossRef CAS.
- G. Wittig and U. Schollkopf, Chem. Ber., 1954, 87, 1318 CrossRef.
- B. E. Maryanoff and A. B. Reitz, Chem. Rev., 1989, 89, 863 CrossRef CAS.
- E. Vedejs and M. Peterson, Top. Stereochem., 1994, 21, 1 CAS.
- K. C. Nicolaou, M. W. Harter, J. L. Gunzner and A. Nadin, Liebigs Ann./Recl., 1997, 7, 1281 Search PubMed.
- P. Zarbin, J. L. Princival, E. de Lima, A. dos Santos, B. Ambrogio and A. de Oliveira, Tetrahedron Lett., 2004, 45, 239 CrossRef CAS PubMed.
- S. M. Mali, A. Bandyopadhyay, S. V. Jadhav, M. G. Kumar and H. N. Gopi, Org. Biomol. Chem., 2011, 9, 6566–6574 CAS.
- H. Pommer, Angew. Chem., 1977, 89, 437 CrossRef CAS.
- H. Pommer, Angew. Chem., Int. Ed. Engl., 1977, 16, 423 CrossRef.
- H. Pommer and C. Thieme, Top. Curr. Chem., 1983, 109, 165 CrossRef CAS.
- J. G. Lombardino, E. H. Wiseman and W. Mclamore, J. Med. Chem., 1971, 14, 1171–1175 CrossRef CAS.
- C. B. Schapira, I. A. Perillo and S. Lamdan, J. Heterocycl. Chem., 1980, 17, 1281–1288 CrossRef CAS.
- M. Zia-ur-Rehman, J. A. Choudary, M. R. J. Elsegood, H. L. Siddiqui and K. M. Khan, Eur. J. Med. Chem., 2009, 44, 1311 CrossRef CAS PubMed.
- E. W. Brooke, S. G. Davies, A. W. Mulvaney, M. Okada, F. Pompeo, E. Sim, R. J. Vickers and I. M. Westwood, Bioorg. Med. Chem. Lett., 2003, 13, 2527–2530 CrossRef CAS.
- R. Robiette, J. Richardson, V. K. Aggarwal and J. N. Harvey, J. Am. Chem. Soc., 2005, 127, 13468–13469 CrossRef CAS PubMed.
- S. Valverde, M. Martin-Lomas, B. Herradon and S. Garcia-Ochoa, Tetrahedron, 1987, 43, 1895 CrossRef CAS.
- J. M. J. Tronchet and B. Gentile, Helv. Chim. Acta, 1979, 62, 2091 CrossRef CAS.
- J. S. Brimacombe, R. Hanna, A. K. M. S. Kabir, F. Bennett and I. D. Taylor, J. Chem. Soc., Perkin Trans. 1, 1986, 815 RSC.
- P. A. Byrne and D. G. Gilheany, J. Am. Chem. Soc., 2012, 134, 9225 CrossRef CAS PubMed.
- J. Wu, D. Li, H. Wu, L. Suna and W.-M. Daia, Tetrahedron, 2006, 62, 4643 CrossRef CAS PubMed.
- S. Hayman and J. H. Kinoshita, J. Biol. Chem., 1965, 240, 877–882 CAS.
- C. L. Motta, S. Sartini, L. Mugnaini, F. Simorini, S. Tliani, S. salerno, A. M. Marini, F. D. Settimo, A. Lavecchia, E. Novellino, M. Cartore, P. Failli and M. Ciuffi, J. Med. Chem., 2007, 50, 4917–4927 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Supplementary Table S1, copies of 1H, 13C, and NOESY NMR spectra and single X-ray crystal structure data. CCDC 982259 and 982260. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ra01016g |
| This journal is © The Royal Society of Chemistry 2014 |