Open Access Article
Open Access ArticleHalophilic polysulfabetaines – synthesis and study of gelation and thermoresponsive behavior†
Vivek Arjunan
Vasantha
*a,
Satyasankar
Jana
a,
Anbanandam
Parthiban
*a and
Julius G.
Vancso
ab
aInstitute of Chemical and Engineering Sciences (ICES), Agency for Science, Technology and Research (A*STAR), 1 Pesek Road, Jurong Island, Singapore 627833. E-mail: vivek_vasantha@ices.a-star.edu.sg; aparthiban@ices.a-star.edu.sg
bMESA+ Research Institute for Nanotechnology, Faculty of Science and Technology, University of Twente, P. O. Box 217, 7500 AE Enschede, The Netherlands
First published on 13th May 2014
Abstract
Polysulfabetaines (PSBs) derived from zwitterionic sulfates (contrary to commonly used polysulfobetaines which are derived from zwitterionic sulfonates) were synthesized for the first time. PSBs dissolved in brine (halophilic), swelled in deionized water and also exhibited reversible and irreversible thermoresponsive behavior. Large differences in interaction with deionized water and brine solution as well as morphology of particles in dispersion was observed between poly[2-(dimethyl(4-vinylbenzyl)ammonio)ethyl sulfate] (PSB 1) and poly[3-(dimethyl(4-vinylbenzyl)ammonio)propyl sulfate] (PSB 2) even though a single methylene (–CH2–) unit was the sole differentiating factor. The gelation and thermoresponsive behaviour observed in PSBs are similar to that of naturally occurring polymers.
Polymers bearing ionic groups such as polyampholytes and zwitterionic polymers have great potential for various applications due to their unique interactions in aqueous solutions.1–3 Polymeric zwitterionic sulfonates also known as polysulfobetaines4–9 are the predominant class of zwitterionic polymers widely reported thus far because of ease of synthesis as well as due to the commercial availability of wide variety of starting materials. In the case of polysulfobetaines, property variations are introduced by way of either changing the number of methylene units between cationic species which is largely ammonium ion and the sulphur atom or by changing the nature of cation forming unit i.e. the tertiary amine or quarternizable amino compound. The former approach is limited in that the number of carbon atoms can only be varied between three and four. However, by changing the nature of cationic moiety, we recently introduced exceptional changes to the nature of polymer in terms solubility and other characteristics.10 There are possibilities to further influence the characteristics of zwitterionic polymers. One such possibility is the zwitterionic polysulfates which can also be called as polysulfabetaines. Unlike the polysulfobetaines where sulphur atom of sulfonate group is directly linked to carbon, in polysulfabetaines, carbon atom is linked to sulphur through oxygen atom (Fig. 1). Theoretically, this additional oxygen atom can be expected to introduce more hydrophilicity to the polymer. The presence of additional heteroatom in the form of oxygen also increases the distance between counterions. Such increase in distance could ultimately influence the nature of ionic interactions.11 Under some circumstances, the interaction may become more of intermolecular in nature than intramolecular.12 As reported herein, this turned out to be true. Substantial difference in properties were observed even between two polymers where a single methylene (–CH2–) unit was the sole distinguishing factor. Though there are many reports on the synthesis of polysulfobetaines, to the best of our knowledge reports on polysulfabetaines are non-existent. Polysulfabetaines exhibit many interesting characteristics as discussed hereunder including dissolution in concentrated sodium chloride solutions and thus the term halophilic (“salt-loving”). It may be noted that microorganisms like bacteria living under harsher conditions such as brine of varying concentration are commonly referred to as halophilic in literature.13–17
The polymerizable sulfabetaine monomers SBM 1 and 2 were prepared in a single step by ring opening reaction18 of cyclic 1,2-ethylene sulfate and 1,3-propylene sulphate (Schemes 1 and S1†) by N-(4-vinylbenzyl)-N,N-dimethylamine, which led to zwitterionic sulfabetaine monomers in good yields (83–87%). To the best of our knowledge, SBM 1 and 2 have not been reported before. SBM 1 and 2 were obtained as powders which were highly hygroscopic and were thoroughly characterized. The 1H-NMR spectrum of SBM 1 and 2 are given in Fig. 2. Table S1† describes the melting point and mass spectroscopic characterization of sulfabetaine monomers SBM 1 and 2.
 | ||
| Fig. 2 1H-NMR spectra of sulfabetaine monomers (SBM) in D2O and corresponding polysulfabetaines (PSB) in (CF3)2–CD–OD. | ||
SBM 1 and 2 were polymerized by free radical polymerization using 4,4′-azobis(4-cyanovaleric acid) (ACVA) at 90 °C in 0.5 M aqueous NaBr solution to yield polysulfabetaines, PSB 1 and 2 respectively. Scheme 1 also shows the polymerization of sulfabetaine monomers. PSB 1 and 2 were thoroughly characterized by spectroscopic techniques. The polymerization conditions are summarized in Table S2.† The complete disappearance of vinyl protons after polymerization can be noticed in Fig. 2 which displays the 1H-NMR spectra of PSB 1 and 2. PSB 1 and 2 were also characterized by FT-IR (Fig. S1†) and UV-Vis (Fig. S2†) spectroscopic techniques. The solubility characteristics of PSB 1 and 2 were determined in various solvents as detailed in Table S3.† PSB 1 and 2 were found to be soluble in formic acid, hexafluoroisopropanol, saturated NaCl solution, trichloroacetic acid and trifluoroethanol. Both PSB 1 and 2 were completely insoluble in common organic solvents like acetic acid, chloroform, methanol as well as in dipolar aprotic solvents like DMF, DMSO, etc.
The presence of directly linked heteroatoms in the from of C–O–S linkage in the case of polysulfabetaines warranted us to determine the hydrolytic stability of polysulfabetaines. Table 1 summarizes the hydrolytic stability of PSB 1 and 2. Polysulfabetaines were heated in deionized water and aqueous solution of 6 M HCl at 95 °C for 24 h in two separate reactions to evaluate hydrolytic stability. The sulphur content of the starting polymers and that of those subjected to hydrolysis were determined by elemental analysis. Since the O–S linkage is expected to cleave upon hydrolysis, the polysulfabetaines undergoing hydrolysis would show lesser (partial hydrolysis) to no or near zero sulphur (substantial to complete hydrolysis) content. As can be noticed from Table 1, the extent of hydrolysis in deionized water was negligible. Even in 6 M HCl acid solution the extent of hydrolysis was about 60% thereby indicating that polysulfabetaines are fairly stable.
| Polymer | Sulfur content (calc.) | Sulfur content (observed) | Sulfur content after treatment with DI watera | Sulfur content after treatment with 6 M HCla |
|---|---|---|---|---|
| a After heating at 95 °C for 24 h. The polymers were dialyzed and lyophilized before elemental analysis. | ||||
| PSB 1 | 11.16 | 10.31 | 10.31 | 4.3 |
| PSB 2 | 10.64 | 10.2 | 9.92 | 4.01 |
Polysulfabetaines, PSB 1 and 2 absorbed water to a varying degree and formed hydrogels. The swelling tendency and the lack of solubility in deionized water in spite of their zwitterionic character are due to strong ionic interactions. The equilibrium swelling and hydration studies of PSBs are summarized in Table 2. The water absorption of PSB 1 was found to be 0.95 g per g of polymer. PSB 2 absorbed significantly higher amount of water (1.97 g per g of polymer) (Table S4†). The difference in water absorbing tendency between PSB 1 and 2 is likely due to the nature of interactions prevailing in the sulfabetaine polymers. The presence of extra methylene unit (–CH2–) could aid in the flexibility as well as separate the charges farther apart. The former effect could help in creating larger ion cages which could accommodate more water. The latter could enhance the interchain interactions which can also create larger void thereby facilitating the binding of more water molecules. PSB 2 also showed increased absorption tendency in 0.9 wt% aqueous NaCl solution. In this case, the absorption was found to be 8 g per g of polymer.
| Sample | Swelling ratioa (SR), % DI H2O | DSCb (Tmax, °C) | Critical salt concentrationc (CSC, wt%) | |
|---|---|---|---|---|
| Titration | Transmittance | |||
| a SR = [(weight of swollen polymer − weight of dry polymer)/weight of dry polymer] × 100. b Endothermic transition signifying the temperature at which expulsion of water from the swollen polymers occurred. c For CSC, 1.11 wt% of PSB 1 and 0.87 wt% of PSB 2 were dissolved in 22.62 wt% of NaCl solution and titrated with deionised water at 25 °C (Fig. S71). | ||||
| PSB 1 | 95.08 ± 4.8 | 58 | 21.04 ± 0.2 (3.59 M) | 21.71 |
| PSB 2 | 197.86 ± 6.7 | 72 | 12.93 ± 0.2 (2.21 M) | 12.09 |
The hydrogel is formed by the polar interaction between zwitterions and water as well as by the presence of water in voids existing in the network formed through ionic interactions. Since heating the gel can disturb these interactions, the behavior of hydrogels was studied by differential scanning calorimetry (DSC) (Fig. S3†). In DSC analysis, PSB 1 hydrogel exhibited an endothermic transition centred around at 58 °C, while PSB 2 showed an endothermic transition at 72 °C. As was also visually observed and will be described later, heating helped the chains to align together closely which in turn enhanced the ionic interactions because of which association with water was disturbed. As a consequence, water was expelled from the network and also induced contrasting morphological changes in the precipitated polymer as observed in a transmission electron microscope (TEM).
In polysulfobetaines derived from methacrylate and acrylamide19–23 the network was gradually destroyed at higher temperature in water and became soluble (UCST). In contrast, PSB gels underwent temperature induced collapse and showed some unexpected morphological changes. Visually, the expulsion of water from PSB hydrogels, as indicated by the formation of deep milky appearance upon heating, occurred, in PSB 1 around 55–60 °C and in PSB 2 around 60–65 °C which are comparable to the transitions observed in DSC. The polymers began to coagulate and precipitate, when the gel was kept for very short time (<1 min) above the cloud point temperature (Fig. 3b). This phenomenon is comparable to the situation prevailing in some biomacromolecules. It is well known that upon thermal treatment biomacromolecules such as gelatin, polysaccharides, collagen and lactoglobulin undergo conformational changes, resulting in the formation of network structures through strong intermolecular interactions.12,24,25
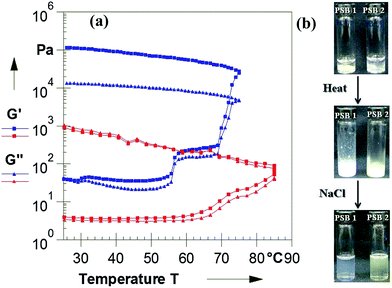
The rheological properties of PSB hydrogels were studied by time, amplitude and frequency sweep measurements (Fig. S4†). Frequency sweep measurements of PSB hydrogels showed that the storage modulus (G′, the elastic response) and the loss modulus (G′′, the viscous response) were dependent over the entire frequency range. The data suggested that the measured G′ exhibited characteristic linear viscoelastic behaviour. However, large variations observed in the curve clearly indicate the difference in nature of interaction between PSB 1 and 2. The result reflected the gel-like behaviour of PSB 1 and 2 and there was no break-up of the gel microstructure.
The irreversible changes that occurred in PSB hydrogels upon heating due to the enhanced ionic interactions between the charged sulfate, and ammonio groups were also evidenced by temperature dependent oscillatory measurement and were consistent with the phase transitions observed visually as well as by DSC. Fig. 3a shows that upon increasing the temperature from 25 to 55 °C, a slight increase of G′ was observed. Above 55 °C, G′ started to increase with temperature indicating that gel network was broken which can be attributed to the expulsion of water. Above 60–75 °C represented the temperature range where G′ modulus increased abruptly upon heating because of the formation of self assembled 3D networks (Fig. 3) of varying dimension and shape as dictated by the nature of basic ionic interactions. Upon increasing the temperature above phase transition, higher values of G′ were reached, indicating the formation of a more stable, irreversible network. Also, the increase in G′ and G′′ after phase transition indicated the existence of a stiffened network. Upon cooling, G and G′′ showed some deviation thereby showing the irreversible behaviour.
The self-assembly26,27 of heat-induced aggregates of PSB was investigated by TEM and dynamic light scattering (DLS) techniques. Fig. 4a and b show TEM images of the PSB gels (1 wt%) after heat treatment at 70 °C for 15 min. It is clear from TEM micrographs that PSBs formed self-assembled aggregates. At 70 °C, PSB 1 formed spherical, predominantly monodispersed aggregates in the range of 70–90 nm (Fig. S5†). The shape of the aggregates obtained for PSB 2, on the other hand, was completely different, rod like aggregates of 300–600 nm with the cross-section of around 100 nm. PSB 2 formed large macro aggregates predominantly through interchain interactions which prolonged to the extent of forming fibrils28 similar to irreversible hydrocolloids like collagens25,29 or carrageen.30 Interestingly, the dendritic micellar microstructures observed in PSB 2 was caused by the presence of one additional methylene unit as compared to PSB 1.
DLS experiments further validated these observations (Fig. S6†). Upon addition of salt to the heat treated dispersions, deep milkiness of the dispersion changed to more translucent in appearance thereby indicating that solvation of zwitterions accompanied by the uncoiling of chains under the influence of charged interaction of the added electrolyte (Fig. 3b). PSB polymer chains folded at higher temperatures due to the prevalence of intra- and interchain ionic interactions and near complete unfolding occurred upon the addition of salt thus showing unusual halophilic behaviour.
The halophilic polysulfabetaines, PSB 1 and 2 also exhibited temperature dependent phase separation in brine solution. The characteristically different states were observed at low (turbid phase; association) and high temperatures (clear phase; dissociation). This association–dissociation behaviour depended on the critical salt concentration31–33 (CSC) or critical electrolyte concentration34 (CEC) (minimum salinity that can cause dissolution) and is summarized in Table 2 (Fig. S7†). Like polysulfobetaines,10,35–37 polysulfabetaines also exhibited reversible UCST behavior in salt solution between 20 °C and 97 °C. This phase transition represents, to the best of our knowledge, the first example of a thermoresponsive UCST behavior in presence of higher salt concentration. As expected, with decrease in salt concentration the UCST increased. The three dimensional plot of the UCST of the PSBs as a function of NaCl and polymer concentrations with respect to temperature is shown in Fig. 5a. As can be inferred from Fig. 5a, the UCST window shifted from low temperature region to high temperature region, simply upon lowering the salt concentration (Fig. S8†). UCST of PSB 1 was higher than that of PSB 2. Furthermore, it was observed that PSB 2 favoured less interaction when compared to its counterpart thereby indicating the prevalence of interchain interactions caused by the additional methylene unit. It may be noted that similar trend was observed in sulphate based low molecular weight surfactants as well.38,39
The reversibility of turbid-to-clear (globular-to-coil conformations) transitions in brine solution upon increasing the temperature was further studied using DLS (Fig. S9†) and viscometry. At the elevated temperature, above the UCST, the PSBs became clear without any observable aggregates (coil form; 25–60 nm) due to charge screening (better solvation thus greater solvent–polymer interaction and reduced polymer–polymer interaction). As the temperature decreased, the polymer conformation changed, resulting in the collapse of the polymer coil which led to larger aggregates (like globular) with the average size above 400 nm (greater polymer–polymer interaction). This resulted in the formation of turbid solution. Heating induces dissociation caused by the mobility of polymer chains. Additionally, the tendency to form aggregates below UCST was also supported by viscosity studies (Fig. S10†). Similar to polysulfobetaines,10 polysulfabetaines also showed non-Newtonian behaviour (Fig. S11†), due to the enhanced chain–chain interactions caused by zwitterionic nature at higher shear rate. Thus, polysulfabetaines exhibited electrolyte induced thermoresponsive behaviour which is accompanied by conformational changes such as globule-to-coil transition.
In summary, we have reported a novel class of halophilic, thermoresponsive polysulfabetaines. The presence of one extra methylene unit between the counterions of polysulfabetaines induced more interchain interactions thereby influenced properties like water uptake, sorption of brine solution, contrasting rheological and thermoresponsive behaviour. Ionic interactions increased with temperature which resulted in the expulsion of water from swollen polysulfabetaine gels. The nature of interactions (intra- vs. inter-) also determined the shape of particles formed which varied from spherical to rod like, dendritic aggregates. Furthermore, polysulfabetaines showed a characteristic and reproducible thermoresponsive behavior in salt solution. UCST behaviour was influenced by the concentration of salt as well as temperature. Such a subtle behavior of polysulfabetaines in brine solution is very interesting and may be suited for a range of applications such as enhanced oil recovery in the petroleum field and low temperature precipitation of proteins in biomedical field.
Acknowledgements
This work was funded by the Agency for Science, Technology and Research (A*STAR), Singapore under Innovative Marine Antifouling Solutions (IMAS) for high-value applications programme. The authors thank Ms. Chen Junhui and Ms. Foo Ming Choo for assisting in GPC and DLS studies and Ms. Tay Boon Ying for assistance with TEM analysis.Notes and references
- A. V. Dobrynin, R. H. Colby and M. Rubinstein, J. Polym. Sci., Part B: Polym. Phys., 2004, 42, 3513–3538 CrossRef CAS.
- S. E. Kudaibergenov and A. Ciferri, Macromol. Rapid Commun., 2007, 28, 1969–1986 CrossRef.
- S. Jana, V. A. Vasantha, L. P. Stubbs, A. Parthiban and J. G. Vancso, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 3260–3273 CrossRef.
- A. B. Lowe and C. L. McCormick, Chem. Rev., 2002, 102, 4177–4189 CrossRef CAS PubMed.
- M. S. Donovan, A. B. Lowe, T. A. Sanford and C. L. McCormick, J. Polym. Sci., Part A: Polym. Chem., 2003, 41, 1262–1281 CrossRef CAS.
- F. Q. Xuan and J. S. Liu, Polym. Int., 2009, 58, 1350–1361 CrossRef CAS.
- S. Y. Jiang and Z. Q. Cao, Adv. Mater., 2010, 22, 920–932 CrossRef CAS PubMed.
- J. Kuang and P. B. Messersmith, Langmuir, 2012, 28, 7258–7266 CrossRef CAS PubMed.
- Z. Cao and S. Jiang, Nano Today, 2012, 7, 404–413 CrossRef CAS PubMed.
- V. A. Vasantha, S. Jana, A. Parthiban and J. G. Vancso, Chem. Commun., 2014, 50, 46–48 RSC.
- R. G. Laughlin, Langmuir, 1991, 7, 842–847 CrossRef CAS.
- B. Ozbas, J. Kretsinger, K. Rajagopal, J. P. Schneider and D. J. Pochan, Macromolecules, 2004, 37, 7331–7337 CrossRef CAS.
- B. van den Burg, Curr. Opin. Microbiol., 2003, 6, 213–218 CrossRef CAS.
- P. DasSarma, J. A. Coker, V. Huse, S. DasSarma and M. C. Flickinger, in Encyclopedia of Industrial Biotechnology, John Wiley & Sons, Inc., 2009 Search PubMed.
- A. Oren, Microbiol. Mol. Biol. Rev., 1999, 63, 334–348 CAS.
- J. M. Pastor, M. Salvador, M. Argandona, V. Bernal, M. Reina-Bueno, L. N. Csonka, J. L. Iborra, C. Vargas, J. J. Nieto and M. Canovas, Biotechnol. Adv., 2010, 28, 782–801 CrossRef CAS PubMed.
- A. Oren, Environ. Technol., 2010, 31, 825–834 CrossRef CAS PubMed.
- D. A. Tomalia and J. C. Falk, J. Heterocycl. Chem., 1972, 9, 891–894 CrossRef CAS.
- J. C. Salamone, W. Volksen, A. P. Olson and S. C. Israel, Polymer, 1978, 19, 1157–1162 CrossRef CAS.
- S. Liang, Q. M. Yu, H. Yin, Z. L. Wu, T. Kurokawa and J. P. Gong, Chem. Commun., 2009, 7518–7520 RSC.
- J. C. Galin and M. Galin, J. Polym. Sci., Part B: Polym. Phys., 1995, 33, 2033–2043 CrossRef CAS.
- J. Ning, G. Li and K. Haraguchi, Macromolecules, 2013, 46, 5317–5328 CrossRef CAS.
- Z. Zhang, T. Chao and S. Jiang, J. Phys. Chem. B, 2008, 112, 5327–5332 CrossRef CAS PubMed.
- M. Meyer, M. Antonietti and H. Schlaad, Soft Matter, 2007, 3, 430–431 RSC.
- H. Hoermann and H. Schlebusch, Biochemistry, 1971, 10, 932–937 CrossRef CAS.
- T. Vermonden, R. Censi and W. E. Hennink, Chem. Rev., 2012, 112, 2853–2888 CrossRef PubMed.
- P. J. Roth, T. P. Davis and A. B. Lowe, Polym. Chem., 2012, 3, 2228–2235 RSC.
- V. J. Bradford and B. L. Iverson, J. Am. Chem. Soc., 2008, 130, 1517–1524 CrossRef CAS PubMed.
- K. E. Kadler, D. F. Holmes, J. A. Trotter and J. A. Chapman, Biochem. J., 1996, 316, 1–11 CAS.
- T. Funami, M. Hiroe, S. Noda, I. Asai, S. Ikeda and K. Nishimari, Food Hydrocolloids, 2007, 21, 617–629 CrossRef CAS PubMed.
- V. M. Monroy Soto and J. C. Galin, Polymer, 1984, 25, 254–262 CrossRef.
- D. A. Z. Wever, F. Picchioni and A. A. Broekhuis, Prog. Polym. Sci., 2011, 36, 1558–1628 CrossRef CAS PubMed.
- K. C. Khilar and H. S. Fogler, J. Colloid Interface Sci., 1984, 101, 214–224 CrossRef CAS.
- E. E. L. Kathmann, L. A. White and C. L. McCormick, Macromolecules, 1997, 30, 5297–5304 CrossRef CAS.
- D. N. Schulz, D. G. Peiffer, P. K. Agarwal, J. Larabee, J. J. Kaladas, L. Soni, B. Handwerker and R. T. Garner, Polymer, 1986, 27, 1734–1742 CrossRef CAS.
- J. Seuring and S. Agarwal, Macromol. Rapid Commun., 2012, 33, 1898–1920 CrossRef CAS PubMed.
- Q. Zhang, J.-D. Hong and R. Hoogenboom, Polym. Chem., 2013, 4, 4322–4325 RSC.
- P. G. Faulkner, A. J. I. Ward and D. W. Osborne, Langmuir, 1989, 5, 924–926 CrossRef CAS.
- P. G. Nilsson, B. Lindman and R. G. Laughlin, J. Phys. Chem., 1984, 88, 6357–6362 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed experimental description and results including 1H NMR, FT-IR DLS, TEM, detailed phase studies etc. See DOI: 10.1039/c4ra00928b |
| This journal is © The Royal Society of Chemistry 2014 |