Synthesis, characterization and gas transport properties of cardo bis(phenylphenyl)fluorene based semifluorinated poly(ether amide)s
Parthasarathi Bandyopadhyay,
Debaditya Bera,
Sipra Ghosh and
Susanta Banerjee*
Material Science Centre, Indian Institute of Technology, Kharagpur – 721302, India. E-mail: susanta@matsc.iitkgp.ernet.in; Fax: +91 3222 255303; Tel: +91 3222 283972
First published on 16th June 2014
Abstract
Here we report the synthesis and properties of a series of processable poly(ether amide)s (PAs) having the bis(phenylphenyl)fluorene moiety prepared from a new diamine monomer, 9,9-bis-[3-phenyl-4-{2′-trifluoromethyl-4′-(4′′-aminophenyl) phenoxy}phenyl]fluorene. Analogous PAs having the bis(phenyl)fluorene moiety have also been prepared from the diamine 9,9-bis-[4-{2′-trifluoromethyl-4′-(4′′-aminophenyl) phenoxy} phenyl]fluorene for comparative purposes. The synthesized PAs exhibited high thermal stability (up to 517 °C and 539 °C in air and nitrogen respectively, for 10% weight loss), high glass transition temperature (272–299 °C) and high tensile strength up to 117 MPa. The optically transparent membranes (λcut-off ≥ 368 nm) from these PAs showed high gas permeability in barrer (PCO2 up to 67.42 and PO2 up to 15) and high permselectivity (PCO2/PCH4 up to 88.37 and PO2/PN2 up to 10.84); especially for the O2/N2 gas pair the PAs surpass or touch the present upper boundary limit of 2008 drawn by Robeson. The effect of the bis(phenylphenyl)fluorene moiety and diacid moieties in PAs on the dielectric values, thermal, mechanical, optical and gas transport properties were correlated.
1. Introduction
Amorphous polyamides have several applications in the area of membrane based gas separation, coatings, engineering plastics, polymer blends, and composites. Aromatic polyamides (high Tg) can be utilized as promising membrane materials in the gas permeation field because of their excellent thermal, mechanical and solvent resistance properties.1–4 There is enormous possibility of preparing a large variety of polyamide structures by suitably designing the diacids or diamine monomers.1–4 However, rigidity of their backbone and interchain hydrogen bonding between polymer repeat unit structures result in very poor processability, high softening temperatures and low gas permeability.1–4 Hence, the major challenge is to synthesize processable PAs without affecting their excellent properties and to improve the permeability and permselectivity. Over the past two decades, membrane based gas separation is an economically and environmentally attractive alternative to the cryogenic or pressure swing adsorption processes.5 The membrane based gas separation processes have gained wide acceptance due to carbon dioxide capture and separation, natural gas purification, and separation of CO2/N2 and produce N2 from air.2,3,5 At present, global warming has been identified as one of the world's major environmental worry. Capture of CO2 especially attained a great importance because of the environmental protection i.e., global warming.6,7 Recently, polymeric gas separation membranes have been used for the capture of carbon dioxide from power plant flue gases.6,7Till now, many polymers have been considered as potential membrane materials but actually few of them found real application in industrial scale.7 The suitable gas separation membranes for industrial gas separating plants should have sufficiently good mechanical and film-forming properties, with a good chemical and thermal stability under the conditions of the separation process.6 The membrane should have sufficient resistance to plasticization and absence of aging i.e., reduction of permeability with respect to time.6 In gas separation application, a tradeoff generally exists between permeability and permselectivity i.e., any improvement in permeability accompanied with a decrease of permselectivity, and vice versa.8 Therefore, the primary aim is to improve gas permeation by overcoming the “tradeoff” behavior between permeability and permselectivity of polymers. Gas permeation through polymer membrane proceeds by solution diffusion mechanism.9 In this process gas molecules get absorbed on the polymeric surface after that move through the polymeric membrane by jumping through the adjacent holes (FFV i.e., fractional free volume) in the polymer due to disruption of the interchain packing. The jump occurs by the transient opening of the leap channels of effective size.
Therefore, the polymer chain mobility (rigidity), inter-chain distance and FFV are the important factors in determining gas transport properties in the solution–diffusion mechanism for the glassy polymers.8 The chain rigidity increases the permselectivity and lowers the permeability. The greater interchain distance between polymer repeat unit structures imparts higher permeability but lower permselectivity.8 Therefore; chain stiffness should be coupled with an increase in interchain separation in order to get simultaneously higher permeability and permselectivity.8 The cardo group, bulky pendant groups, kinks and bends in the polymeric structures inhibit the close packing, leading to higher fractional free volume (FFV) which, in turn, improve the processability and gas permeability.1–5,10,11
New classes of partially fluorinated polymers are of special interest because of their possible use as gas permeation membranes and their enhanced flame resistance, low dielectric constant, and remarkably low water absorption values.10–12 Perfluorinated polymeric membranes showed low tendency of plasticization in the presence of hydrocarbon vapors, common impurities in gas mixtures in gas permeation study.6 The trifluoromethyl groups (–CF3) in the polymer backbone improve the polymer solubility which is known as fluorine effect without penalty of thermal stability.12 The bulky –CF3 group also serves to increase the free volume and rigidity of the polymer, thereby improving gas permeabilities and permselectivity values simultaneously.12
The bis(phenyl)fluorene-based cardo polymers have a structure in which a bulky fluorene moiety projects vertically from the polymer main chain.5 The four phenyl rings connected to a quaternary carbon centre in cardo bis(phenyl)fluorene moiety experience severe rotational hindrance. Therefore, the cardo moiety must reduce the packing as well as increase the rigidity of main chains which, in turn, improve the gas permeability and permselectivity values.5,11 In recent years, polymers containing fluorene moieties have been extensively studied because of their potential applications as photoelectronic materials and show high thermal stability, enhanced solubility, low refractive index, high optical transparency with low dielectric constant.5,11,13–15
Bis(phenylphenyl)fluorene based polymers are expected to be highly sterically hindered due to two additional pendant phenyl rings in comparison to the analogous bis(phenyl)fluorene based polymers. Therefore, the study of gas separation properties for such analogous polymers having bis(phenylphenyl)fluorene and bis(phenyl)fluorene moieties and comparison of their properties will be interesting. Therefore in continuation of our investigation on new polymeric membranes for superior gas separation performance, in the present investigation we would like to report the synthesis, characterization and gas transport study of a series of new poly(ether amide) (PA) membranes containing bis(phenylphenyl)fluorene moiety. Besides, this work focused to understand the relationship between the structural variations in the repeat unit of the PAs and their physical properties and gas permeability/selectivity. Finally, the gas transport properties of these polyamides have been compared with structurally analogues polymers having bis(phenylfluorene) moiety.
2. Experimental
2.1. Equipments
Carbon, hydrogen and nitrogen content of the compounds were determined by pyrolysis method by Vario EL elemental analyzer. 1H-NMR and proton decoupled 13C-NMR spectra were recorded on a Bruker 200, 400 and 600 MHz instrument (Switzerland) using CDCl3 (deuterated chloroform) or DMSO-d6 (deuterated dimethyl sulfoxide) or pyridine-d5 (deutered pyridine) as solvents. FTIR spectra of the polymer membranes were recorded with a NEXUS Nicolet Impact-410 spectrophotometer. Gel permeation chromatography (GPC) was done with a Waters instrument (Waters 2414). Tetrahydrofuran (THF) was used as eluent (flow rate 0.5 mL min−1, polystyrene was used as standard and RI detector was used to record the signal) and Styragel HR-4 columns were employed. NETZSCH DSC 200PC differential scanning calorimeter (DSC) was used (heating rate of 20 °C min−1) to measure the glass transition temperature of the polymers in nitrogen. Glass transition temperatures (Tg) were taken at the midpoint of the step transition in the second heating run. Thermal decomposition behavior of these polymers was investigated using a NETZSCH TG 209 F1 instrument at a heating rate of 10 °C min−1 under synthetic air (N2–O2 is 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) and nitrogen. Tensile strength and elongation at break of the thin polyamide membranes were measured with the help of UTM-INSTRON, PLUS, Model no. 8800. Test samples with dimension of 10 × 25 mm2 and a thickness around 80–85 μm were used for the measurement of tensile strength and percentage of elongation at break. Tests were done using a cross-head speed of 5% min−1 of the specimen length. Ultra violet spectra of the polymer films were recorded at room temperature using detector SD-2000 (Ocean Optics Inc.) and source lamp DH-2000. The dielectric constant values of the polyamide films were determined by the parallel plate capacitor method with HIOKI 3532-50 LCR Hi Tester at 1 MHz at a temperature of 30 °C and a relative humidity of 45. Wide angle X-ray diffractograms were recorded by Rigaku, Ultema III X-ray diffractometer using a Cu Kα (0.154 nm) source. The Cu Kα source operated at 40 kV and 40 mA and the range of 2 theta for XRD measurement was 10–40°. The densities of the membranes were measured using a Wallace High Precision Densimeter-X22B (UK) (isooctane displacement) at 30 °C. The gas transport properties of the PA membranes were studied at 3.5 bar of applied gas pressure and at 35 °C using automated Diffusion Permeameter (DP-100-A) manufactured by Porous Materials Inc., USA. Ultrahigh pure (99.99%) gases (methane, nitrogen, oxygen, and carbon dioxide) from BOC Gases, India, were used for the permeation study. The permeability coefficient, ideal perm selectivity (α), diffusion coefficient (D), ideal diffusivity selectivity (αD), solubility coefficient (S), and ideal solubility selectivity (αS) were determined according to reported procedure.3,10 The reproducibility of the measurements was checked from three independent measurements using the same membrane and it was better than ±5%.
20) and nitrogen. Tensile strength and elongation at break of the thin polyamide membranes were measured with the help of UTM-INSTRON, PLUS, Model no. 8800. Test samples with dimension of 10 × 25 mm2 and a thickness around 80–85 μm were used for the measurement of tensile strength and percentage of elongation at break. Tests were done using a cross-head speed of 5% min−1 of the specimen length. Ultra violet spectra of the polymer films were recorded at room temperature using detector SD-2000 (Ocean Optics Inc.) and source lamp DH-2000. The dielectric constant values of the polyamide films were determined by the parallel plate capacitor method with HIOKI 3532-50 LCR Hi Tester at 1 MHz at a temperature of 30 °C and a relative humidity of 45. Wide angle X-ray diffractograms were recorded by Rigaku, Ultema III X-ray diffractometer using a Cu Kα (0.154 nm) source. The Cu Kα source operated at 40 kV and 40 mA and the range of 2 theta for XRD measurement was 10–40°. The densities of the membranes were measured using a Wallace High Precision Densimeter-X22B (UK) (isooctane displacement) at 30 °C. The gas transport properties of the PA membranes were studied at 3.5 bar of applied gas pressure and at 35 °C using automated Diffusion Permeameter (DP-100-A) manufactured by Porous Materials Inc., USA. Ultrahigh pure (99.99%) gases (methane, nitrogen, oxygen, and carbon dioxide) from BOC Gases, India, were used for the permeation study. The permeability coefficient, ideal perm selectivity (α), diffusion coefficient (D), ideal diffusivity selectivity (αD), solubility coefficient (S), and ideal solubility selectivity (αS) were determined according to reported procedure.3,10 The reproducibility of the measurements was checked from three independent measurements using the same membrane and it was better than ±5%.
2.2. Starting materials
9-Fluorenone, 3-mercaptopropionic acid, 2-phenylphenol, 4,4′-(hexafluoroisopropylidene)bis(benzoic acid), 5-tert-butyl-isophthalic acid, isophthalic acid, terephthalic acid, naphthalene-2,6-dicarboxylic acid and tetrakis (triphenyl phosphine) palladium(0) (99%) were purchased from Sigma Aldrich (USA) and triphenyl phosphite (TPP), (DMAc), sulfuric acid (96%), were purchased from E. Merck, India and were used as received. 1-Methyl-2-pyrrolidinone (NMP) (E. Merck, India) was purified by stirring with NaOH and distilled from P2O5 prior to use. Toluene (E. Merck, India) was dried by refluxing over sodium metal. Pyridine (E. Merck, India) was purified by stirring with NaOH and distilled under reduced pressure. CaCl2 (E. Merck, India) and anhydrous K2CO3 (E. Merck, India) were dried for 12 h at 120 °C prior to use. Methanol (Rankem, India) was used for precipitation of polymers. The compound 4-fluoro-4′-nitro-3-trifluoromethyl-biphenyl (3) was prepared according to the procedure reported earlier.16 The 9,9-bis(4-hydroxy-3-phenylphenyl)fluorene (2) was synthesized starting from 9-fluorenone (1) and 2-phenylphenol, in presence of H2SO4, toluene and 3-mercaptopropionic acid, according to procedure reported in literature.17The di(ether amine) ‘5’ monomer was prepared from 9,9-bis(4-hydroxy-3-phenylphenyl)fluorene monomer and depicted in Scheme 1.
2.3. Monomer synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v, 80 mL each). At first, the reaction mixture was heated for 4 h at 140 °C followed by heating at 170 °C for another 2 h. After cooling to the room temperature the reaction mixture was poured slowly into large excess of water. The obtained precipitate was filtered, washed with water for several times and dried under vacuum at 80 °C for overnight.
1, v/v, 80 mL each). At first, the reaction mixture was heated for 4 h at 140 °C followed by heating at 170 °C for another 2 h. After cooling to the room temperature the reaction mixture was poured slowly into large excess of water. The obtained precipitate was filtered, washed with water for several times and dried under vacuum at 80 °C for overnight.Yield: 9 g (∼87%). Anal. calcd for C63H38F6N2O6 (1032.98 g mol−1): C, 73.25%; H, 3.71%; N, 2.71%; found: C, 73.20%; H, 3.76%; N, 2.66%. FTIR (KBr, cm−1): 1606 (aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C ring stretching), 1482, 1341 (asymmetric COC), 1517, 1341 (symmetric and asymmetric NO2 stretch) 1138 (C–F), 1048 (symmetric COC), 899 (CN stretch for aromatic NO2), 825 (aromatic CH bend out of plane). 1H-NMR: δH(ppm) (200 MHz; CDCl3; Me4Si): 8.28 (d, J = 8.6 Hz, 4H, H1), 7.85–7.81 (m, 4H, H4, H15), 7.65–7.54 (m, 6H, H2, H3), 7.47–7.37 (m, 8H, H12, H14, H9), 7.33–7.27 (m, 12H, H7, H8, H13, H10, H11), 6.98 (d, J = 8.6 Hz, 2H, H6), 6.80 (d, J = 8.6 Hz, 2H, H5).
C ring stretching), 1482, 1341 (asymmetric COC), 1517, 1341 (symmetric and asymmetric NO2 stretch) 1138 (C–F), 1048 (symmetric COC), 899 (CN stretch for aromatic NO2), 825 (aromatic CH bend out of plane). 1H-NMR: δH(ppm) (200 MHz; CDCl3; Me4Si): 8.28 (d, J = 8.6 Hz, 4H, H1), 7.85–7.81 (m, 4H, H4, H15), 7.65–7.54 (m, 6H, H2, H3), 7.47–7.37 (m, 8H, H12, H14, H9), 7.33–7.27 (m, 12H, H7, H8, H13, H10, H11), 6.98 (d, J = 8.6 Hz, 2H, H6), 6.80 (d, J = 8.6 Hz, 2H, H5).
The completion of the reaction was monitored by thin layer chromatography (TLC) and the reaction mixture was filtered at hot condition to remove catalyst. THF was distilled off from the reaction mixture by rotary evaporator. Remaining mixture was poured into 1 L deionised water to precipitate a yellowish solid product. Precipitate was filtered and washed with deionised water to remove entrapped hydrazine. The crude product was dried under vacuum at 60 °C. Pure amine was obtained after purification by silica gel column chromatography using dichloromethane as eluent.
Yield: 7 g (∼74%). Anal. calcd for C63H42F6N2O6 (972.32 g mol−1): C, 77.77%; H, 4.35%; N, 2.88%; found: C, 77.70%; H, 4.40%; N, 2.84%. FTIR (KBr, cm−1): 3469, 3382 (NH stretching), 1621 (aromatic C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C ring stretching), 1480, 1326 (asymmetric COC), 1240, 1130 (CF), 1052 (symmetric COC), 818 (aromatic CH bend out of plane). 1H-NMR: δH(ppm) (400 MHz; DMSO-d6; Me4Si): 7.92 (d, J = 7.2 Hz, 2H, H15), 7.71 (s, 2H, H3), 7.62–7.58 (m, 4H, H4, H12), 7.40–7.33 (m, 6H, H8, H9), 7.29–7.27 (m, 10H, H10, H11, H13, H14), 7.24–7.23 (m, 6H, H7, H2), 6.97–6.95 (m, 2H, H6), 6.80 (d, J = 8.4 Hz, 2H, H5), 6.62 (d, J = 8.4 Hz, 4H, H1). 13C-NMR: δC(ppm) (150 MHz; DMSO-d6; Me4Si): 153.12, 151.67, 150.95, 149.42, 142.71, 140.24, 137.27, 136.42, 133.28, 131.42, 130.74, 129.80, 129.42, 128.94, 128.71, 128.26, 127.85, 126.81, 125.92, 125.06, 124.08, 123.25, 121.44, 120.64, 119.98 (q, J = 30 Hz), 119.20, 114.97, 64.79. 19F-NMR: δF(ppm) (376 MHz; DMSO-d6,; CFCl3): −61.29 (s).
C ring stretching), 1480, 1326 (asymmetric COC), 1240, 1130 (CF), 1052 (symmetric COC), 818 (aromatic CH bend out of plane). 1H-NMR: δH(ppm) (400 MHz; DMSO-d6; Me4Si): 7.92 (d, J = 7.2 Hz, 2H, H15), 7.71 (s, 2H, H3), 7.62–7.58 (m, 4H, H4, H12), 7.40–7.33 (m, 6H, H8, H9), 7.29–7.27 (m, 10H, H10, H11, H13, H14), 7.24–7.23 (m, 6H, H7, H2), 6.97–6.95 (m, 2H, H6), 6.80 (d, J = 8.4 Hz, 2H, H5), 6.62 (d, J = 8.4 Hz, 4H, H1). 13C-NMR: δC(ppm) (150 MHz; DMSO-d6; Me4Si): 153.12, 151.67, 150.95, 149.42, 142.71, 140.24, 137.27, 136.42, 133.28, 131.42, 130.74, 129.80, 129.42, 128.94, 128.71, 128.26, 127.85, 126.81, 125.92, 125.06, 124.08, 123.25, 121.44, 120.64, 119.98 (q, J = 30 Hz), 119.20, 114.97, 64.79. 19F-NMR: δF(ppm) (376 MHz; DMSO-d6,; CFCl3): −61.29 (s).
2.4. Polymerization and membrane preparation
The di-amine monomer (5) was polymerized with five different aromatic diacids in molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 using NMP as solvent in the presence of triphenyl phosphite (TPP), CaCl2 and pyridine (Scheme 2). A representative polymerization reaction of diamine monomer with 4,4′-(hexafluoroisopropylidene)bis(benzoic acid) is as follows:
1 using NMP as solvent in the presence of triphenyl phosphite (TPP), CaCl2 and pyridine (Scheme 2). A representative polymerization reaction of diamine monomer with 4,4′-(hexafluoroisopropylidene)bis(benzoic acid) is as follows:
a mixture of diamine (5), 9,9-bis-[3-phenyl-4-{2′-trifluoromethyl-4′-(4′′-aminophenyl) phenoxy}phenyl]fluorene (0.712547 g, 0.73283 mmol), 4,4′-(hexafluoroisopropylidene)bis(benzoic acid) (0.287453 g, 0.73283 mmol), calcium chloride (0.36 g), NMP (5 mL), pyridine (1.4 mL), and TPP (1.4 mL, 5.34 mmol) were taken in a 50 mL round bottom flask equipped with reflux condenser. The mixture was heated with continuous stirring (using magnetic stirrer) at 110 °C for 6 h under nitrogen atmosphere. The reaction mixture became highly viscous during this period.
The mixture was poured in methanol (500 mL) with constant stirring and obtained a precipitate off white fibrous polymer. The polymer was washed thoroughly with distilled water (hot and cold) followed by methanol for complete removal of any traces of solvent and CaCl2. The off-white fibrous polymer was dried overnight at 80 °C in a vacuum oven. The same method was used for the preparation of other PAs.
The analogous five PAs were prepared from the diamine monomer 9,9-bis-[4-{2′-trifluoromethyl-4′-(4′′-aminophenyl) phenoxy} phenyl]fluorene on reaction with five different aromatic dicarboxylic acids by similar phosphorylation reaction. The PAs prepared from this diamine monomer with 5-tert-butyl-isophthalic acid, isophthalic acid and terephthalic acid respectively were reported earlier.18–20
The polymeric membranes were prepared by casting 10–15% (w/v) homogeneous polymer solutions in DMAc solvent onto clean glass Petri dishes according to our previous reported protocol.2,3
The thicknesses of the membranes were between 75–80 μm. The membranes were employed for elemental analyses, solubility tests, density determination, thermal analyses, tensile tests, X-ray diffraction measurements, gas permeability measurements and to record the ATR-FTIR and NMR spectra. The specific density values were used to determine the fractional free volume (FFV) of the polymers for each gas, using group contribution method developed by Park and Paul.21 The polymer/membrane properties are reported in Tables 1–3.
| Polymer | Mna (g mol−1) | PDIb | λ0c (nm) | UV-transmittanced (%) | Dielectric constant (1 MHz) | dspe (Å) |
|---|---|---|---|---|---|---|
| a Number average molecular weight; polystyrene calibration.b Polydispersity index.c Cut-off wave length.d UV-transmittance at 500 nm (%).e dsp: intersegmental distance between the polymer chain obtained from the Bragg equation. | ||||||
| PA ‘A’ | 106![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2.2 | 371 | 68 | 2.41 | 5.99 |
| PA ‘B’ | 122![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2.1 | 374 | 79 | 2.20 | 6.13 |
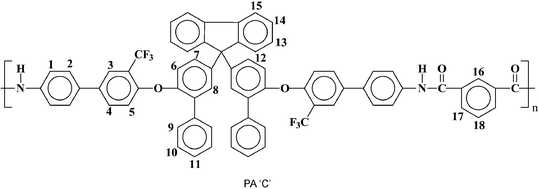
| PA ‘C’ | 102![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2.5 | 378 | 64 | 2.59 | 5.89 |
| PA ‘D’ | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
2.0 | 399 | 67 | 2.55 | 5.82 |
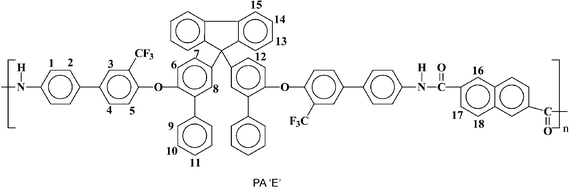
| PA ‘E’ | 172![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2.7 | 403 | 41 | 2.65 | 5.55 |
| PA ‘A′’ | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2.8 | 368 | 68 | 2.51 | 5.95 |
| PA ‘B′’ | 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
2.5 | 381 | 70 | 2.40 | 6.08 |
| PA ‘C′’ | 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2.5 | 382 | 61 | 3.31 | 5.84 |
| PA ‘D′’ | 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2.7 | 403 | 62 | 2.96 | 5.58 |
| PA ‘E′’ | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 850 850 |
2.3 | 407 | 40 | 2.80 | 5.41 |
| Polymer | Densitya | N–H group density (%) | FFV (CH4) | FFV (N2) | FFV (O2) | FFV (CO2) |
|---|---|---|---|---|---|---|
| a Density (g cm−3) measured at 30 °C. | ||||||
| PA ‘A’ | 1.22 | 2.59 | 0.188 | 0.171 | 0.176 | 0.178 |
| PA ‘B’ | 1.17 | 2.26 | 0.195 | 0.190 | 0.191 | 0.194 |
| PA ‘C’ | 1.24 | 2.72 | 0.164 | 0.151 | 0.154 | 0.159 |
| PA ‘D’ | 1.27 | 2.72 | 0.159 | 0.146 | 0.149 | 0.154 |
| PA ‘E’ | 1.29 | 2.60 | 0.133 | 0.125 | 0.129 | 0.133 |
| PA ‘A′’ | 1.26 | 2.98 | 0.179 | 0.164 | 0.168 | 0.167 |
| PA ‘B′’ | 1.21 | 2.55 | 0.179 | 0.177 | 0.177 | 0.178 |
| PA ‘C′’ | 1.27 | 3.15 | 0.161 | 0.150 | 0.153 | 0.155 |
| PA ‘D′’ | 1.31 | 3.15 | 0.153 | 0.142 | 0.145 | 0.147 |
| PA ‘E′’ | 1.32 | 3.85 | 0.131 | 0.126 | 0.130 | 0.130 |
| –Ar– | –R– | Polymer | Tga (° C) | Td10b (° C) | TSc (Mpa) | Modd (GPa) | EBe (%) | |
|---|---|---|---|---|---|---|---|---|
| In air | In N2 | |||||||
| a Glass transition temperature determined by DSC, heating rate at 20 °C min−1.b 10% degradation temperature measured by TGA under air and nitrogen, heating rate at 10 °C min−1.c Tensile strength.d Young's modulus.e Percent of elongation at break.f Values taken from ref. 18.g Values taken from ref. 19.h Values taken from ref. 20. | ||||||||
 |
 |
PA ‘A’ | 274 | 477 | 517 | 83 | 2.02 | 5.60 |
| Do |  |
PA ‘B’ | 274 | 505 | 520 | 90 | 2.12 | 6.0 |
| Do |  |
PA ‘C’ | 272 | 505 | 527 | 79 | 1.74 | 6.0 |
| Do |  |
PA ‘D’ | 273 | 517 | 536 | 103 | 2.29 | 8.6 |
| Do |  |
PA ‘E’ | 278 | 506 | 539 | 107 | 2.34 | 7.4 |
 |
 |
PA ‘A′’ | 273f | 448f | 481 | 69f | 1.30f | 9.0f |
| Do |  |
PA ‘B′’ | 297 | 497 | 522 | 92 | 1.74 | 11.0 |
| Do |  |
PA ‘C′’ | 285g | 465g | 437 | 71g | 1.8g | 10g |
| Do |  |
PA‘D′’ | 294h | 477h | 440 | 99h | 2.2h | 12.9h |
| Do |  |
PA ‘E′’ | 299 | 468 | 442 | 117 | 2.2 | 11.0 |
Anal. calcd for C75H52F6N2O4 (1159.22 g mol−1): C, 77.71%; H, 4.52%; N, 2.42%; found: C, 77.65%; H, 4.46%; N, 2.47%. FTIR (KBr, cm−1): 3305 (NH), 3053, 2965 (aromatic CH), 2965 (aliphatic CH), 1665 (CO), 1234, 1124 (CF). 1H-NMR: δH(ppm) (400 MHz; pyridine-d5; Me4Si): 11.48 (s, 2H, amide protons), 9.03 (s, 1H, H16), 8.45 (s, 2H, H17), 8.27 (d, J = 8.4 Hz, 4H, H1), 8.01 (d, J = 8 Hz, 2H, H15), 7.95 (s, 2H, H3), 7.90–7.88 (m, 4H, H2), 7.71–7.59 (m, 12H, H4, H14, H8, H9, H12), 7.45–7.41 (m, 4H, H10), 7.35–7.31 (m, 4H, H13, H11), 7.25 (m, 4H, H7, H6), 6.96 (d, J = 8.4 Hz, 2H, H5), 1.14 (s, 9H, H18). 19F-NMR: δF(ppm) (376 MHz; pyridine-d5; CFCl3): −62.88 (s).
Anal. calcd for C80H48F12N2O4 (1329.23 g mol−1): C, 72.29%; H, 3.64%; N, 2.11%; found: C, 72.33%; H, 3.70%; N, 2.06%. FTIR (KBr, cm−1): 3304 (NH), 3059, 2923 (aromatic CH), 1667 (CO), 1241, 1134 (CF). 1H-NMR: δH(ppm) (400 MHz; pyridine-d5; Me4Si): 11.38 (s, 2H, amide protons), 8.26–8.25 (m, 8H, H1, H16), 8.02–8.00 (m, 2H, H15), 7.97 (s, 2H, H3), 7.91–7.89 (m, 4H, H2), 7.72–7.62 (m, 12H, H4, H17, H12, H9), 7.55 (d, J = 7.6 Hz, 4H, H8, H14), 7.48–7.40 (m, 4H, H10), 7.35–7.32 (m, 4H, H13, H11), 7.26–7.24 (m, 4H, H7, H6), 6.96 (d, J = 8.4 Hz, 2H, H5). 19F-NMR: δF(ppm) (376 MHz; pyridine-d5; CFCl3): −62.77 (s), −64.69 (s).
Anal. calcd for C71H44F6N2O4 (1103.11 g mol−1): C, 77.31%; H, 4.02%; N, 2.54%; found: C, 77.26%; H, 4.07%; N, 2.60%. FTIR (KBr, cm−1): 3304 (NH), 3060, 2923 (aromatic C–H), 1662 (CO), 1234, 1123 (CF). 1H-NMR: δH(ppm) (400 MHz; pyridine-d5; Me4Si): 11.30 (s, 2H, amide protons), 9.11 (s, 1H, H16), 8.37 (d, J = 7.2 Hz, 2H, H17), 8.23 (d, J = 8 Hz, 4H, H1), 8.01 (d, J = 7.2 Hz, 2H, H15), 7.96 (s, 2H, H3), 7.89 (m, 4H, H2), 7.71 (d, J = 7.6 Hz, 4H, H4, H12), 7.68–7.62 (m, 6H, H9, H14), 7.48–7.42 (m, 6H, H10, H13), 7.35–7.32 (m, 5H, H8, H11, H18), 7.26–7.24 (m, 4H, H6, H7), 6.97 (d, J = 8.4 Hz, 2H, H5). 19F-NMR: δF(ppm) (376 MHz; pyridine-d5; CFCl3): −62.69 (s).
Anal. calcd for C71H44F6N2O4 (1103.11 g mol−1): C, 77.31%; H, 4.02%; N, 2.54%; found: C, 77.36%; H, 3.99%; N, 2.50%. FTIR (KBr, cm−1): 3288 (NH), 2921, 2851 (aromatic CH), 1659 (CO), 1233, 1123 (CF). 1H-NMR: δH(ppm) (400 MHz; pyridine-d5; Me4Si): 11.33 (s, 2H, amide protons), 8.38 (s, 4H, H16), 8.23 (d, J = 8.8 Hz, 4H, H1), 8.01 (d, J = 7.2 Hz, 2H, H15), 7.95 (s, 2H, H3), 7.91–7.89 (m, 4H, H2), 7.70 (d, J = 8 Hz, 6H, H4, H12, H14), 7.64 (d, J = 8.8 Hz, 6H, H9, H8), 7.45–7.41 (m, 4H, H10), 7.35–7.31 (m, 4H, H11, H13), 7.26–7.22 (m, 4H, H6, H7), 6.95 (d, J = 8 Hz, 2H, H5). 19F-NMR: δF(ppm) (376 MHz; pyridine-d5; CFCl3): −62. 49 (s).
Anal. calcd for C71H44F6N2O4 (1153.17 g mol−1): C, 78.12%; H, 4.02%; N, 2.43%; found: C, 78.07%; H, 4.07%; N, 2.48%. FTIR (KBr, cm−1): 3302 (NH), 3059, 2922 (aromatic CH), 1659 (CO), 1233, 1123 (CF). 1H-NMR: δH(ppm) (400 MHz; pyridine-d5; Me4Si): 11.39 (s, 2H, amide protons), 8.79 (s, 2H, H16), 8.39 (d, J = 7.6 Hz, 2H, H17), 8.32 (d, J = 7.2 Hz, 4H, H1), 8.03–7.97 (m, 6H, H3, H15, H18), 7.92–7.90 (m, 4H, H2), 7.73–7.64 (m, 10H, H4, H9, H12, H14), 7.47–7.43 (m, 4H, H10), 7.37–7.33 (m, 4H, H8, H13), 7.27–7.25 (m, 6H, H11, H6, H7), 6.99 (d, J = 7.6 Hz, 2H, H5). 19F-NMR: δF(ppm) (376 MHz; pyridine-d5; CFCl3): −62.88 (s).
Anal. calcd for C68H40F12N2O4 (1177.04 g mol−1): C, 69.39%; H, 3.43%; N, 2.38%; found: C, 69.34%; H, 3.48%; N, 2.34%. FTIR (KBr, cm−1): 3335 (NH), 3039 (aromatic CH), 1670 (CO), 1237, 1124 (CF). 1H-NMR: δH(ppm) (400 MHz; DMSO-d6; Me4Si): 10.57 (s, 2H, amide protons), 8.08 (d, J = 8.6 Hz, 4H, H12), 7.98–7.89 (m, 10H, H1, H3, H4, H11), 7.72 (d, 4H, J = 8.2 Hz, H2), 7.57–7.49 (m, 6H, H8, H13), 7.44–7.36 (m, 4H, H9, H10), 7.24–7.20 (m, 4H, H7), 7.13–7.01 (m, 6H, H5, H6). 19F-NMR: δF(ppm) (376 MHz; DMSO-d6,; CFCl3): −62.83 (s), −64.76 (s).
Anal. calcd for C63H38F6N2O4 (1000.98 g mol−1): C, 75.59%; H, 3.83%; N, 2.80%; found: C, 75.54%; H, 3.79%; N, 2.85%. FTIR (KBr, cm−1): 3334 (NH), 3037 (aromatic CH), 1654 (CO), 1234, 1124 (CF). 1H-NMR: δH(ppm) (400 MHz; pyridine-d5; Me4Si): 11.44 (s, 2H, amide protons), 8.82 (s, 2H, H12), 8.42–8.36 (m, 6H, H1, H13), 8.09 (s, 2H, H3), 8.00–7.96 (m, 4H, H11, H14), 7.80–7.78 (m, 6H, H2, H4), 7.69 (d, J = 7.6 Hz, 2H, H8), 7.51–7.41 (m, 8H, H7, H9, H10), 7.19–7.10 (m, 6H, H6, H5). 19F-NMR: δF(ppm) (376 MHz; pyridine-d5; CFCl3): −62. 55 (s).
3. Results and discussion
3.1. Monomer synthesis
The fluorinated bis(ether amine) monomer (5) was synthesized starting from 9-fluorenone (1) as shown in Scheme 1. The compound 9,9-bis(4-hydroxy-3-phenylphenyl)fluorene (2) was prepared from 9-fluorenone as reported earlier.17 The dinitro compound (4) was formed by aromatic nucleophilic substitution reaction between the dihydroxy compound (2) and 4-fluoro-4′-nitro-3-trifluoromethyl-biphenyl (3).16 Finally, the dinitro compound was reduced to diamine compound (5) using hydrazine monohydrate and Pd/C as catalyst and THF as solvent. The crude diamino compund was purified through silica gel column chromatography using dichloromethane as eluent.3.2. Polymer synthesis and their properties
The polyamides having bis(phenylphenyl)fluorene moiety (PA ‘A’–‘E’) were synthesized by the polycondensation reaction of the diamine monomer, 9,9-bis-[3-phenyl-4-{2′-trifluoromethyl-4′-(4′′-aminophenyl) phenoxy}phenyl]fluorene with five different aromatic diacids. Similarely, the analogous PAs (PA ‘A′’–‘E′’) having bis(phenyl)fluorene moiety were prepared using the diamine monomer, 9,9-bis-[4-{2′-trifluoromethyl-4′-(4′′-aminophenyl) phenoxy} phenyl]fluorene. All polyamides (PA ‘A’–‘E’) were readily dissolved (10% w/v) at room temperature in NMP, DMF, DMAc, THF and Pyridine. The rate of dissolution in different solvents of bis(phenylphenyl)moiety containing PAs were relatively higher than the analogous bis(phenyl)fluorene moiety containing PAs. This is attributed to the two phenyl groups which reduce the interchain packing in bis(phenylphenyl) fluorene based PAs. Transparent and flexible membranes from these PAs obtained from their DMAc solution.The chemical structures of the polymer repeat units were confirmed by means of elemental analysis, FTIR-ATR, and 1H-NMR spectroscopy. FTIR-ATR spectra of the poly(ether amide)s displayed characteristic absorption bands for amide group in the range of 3305–3288 cm−1 (N–H stretching) and 1656–1665 cm−1 (carbonyl group stretching). The absence of free amine absorption peak above 3400 cm−1 supported high conversion of diamines to polyamides. The results of the elemental analysis (C, H, and N) of the PAs were in good agreement with polymer repeat unit structures.
The repeat unit structures of the polymers were confirmed by the 1H-NMR and 13C-NMR spectra. The PAs showed exact number of desired 1H and 13C signals corresponding to their structures. A representative 1H-NMR spectrum of PA ‘A’ in pyridine-d5 is shown in Fig. 1. The PAs showed a singlet above at 11 ppm corresponding to the amide proton and all the other magnetically different protons, their splitting pattern and relative peak intensities were in accordance to the chemical structure of the polymers.
 | ||
| Fig. 1 1H-NMR spectrum of PA ‘A’ in pyridine-d5 (* signals at 8.74, 7.59, and 7.22 ppm are for pyridine). | ||
In proton decoupled 13C-NMR spectra the C–F (–CF3) coupling constant values for each quartet for one bond coupling (1JC–F) were around 271 Hz and for the two bonds coupling (2JC–F) were around 31 Hz. The peak (δ) for carbonyl carbon appeared at around 166.5 ppm. A representative 13C-NMR spectrum of PA ‘A’ in pyridine-d5 is shown in Fig. 2.
 | ||
| Fig. 2 13C-NMR spectrum of PA ‘A’ in pyridine-d5 (* signals at 150.2, 135.8, and 123.7 ppm are for pyridine). | ||
The PAs showed a singlet for quaternary carbon centre in cardo fluorene moiety at around 65.4 ppm. The peaks corresponds to the aliphatic primary (31 ppm) and tertiary(35 ppm) carbons were found in case of PA ‘A’. All the structural characterization confirmed high conversion of diamine and diacid to polyamide. High molecular weight of the PAs from the GPC results (Mn = 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500–172
500–172![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) also established the above fact.
000 g mol−1) also established the above fact.
UV-visible spectroscopic studies of the PA ‘A’–‘E’ membranes indicated high optical transparency (upto 79% at 500 nm) and a lower cut-off wavelength between 371–403 nm [Table 1]. The lower cut-off wavelength values and high optiacl transparecy (except PA ‘E’) values might be due to the bis(phenylphenyl)fluorene moiety, ether linkages and bulky –CF3 groups in the diamine moiety which reduce the intermolecular interaction between the polymer chains.5,22,23 Highest optical transparency and lower cutoff wavelength (except PA ‘A’) of PA ‘B’ was attributed to the weak intermolecular cohesive interaction between polymer chains due to its hexafluoroisopropylidene group (higher fluorine loading).22,23 The two phenyl groups in bis(phenylphenyl)fluorene moiety undergo steric hindrance and disturbed the conjugation along the backbone. Therefore these polymers showed higher optical transparency and lower cut-off wavelength than analogous polymers. The dielectric constant values of the PA ‘A’–‘E’ membranes were measured at 1 MHz and at a temperature of 30 °C with a relative humidity of 45%. The PAs showed low dielectric constant values [Table 1]. The very low polarizability of C–F bonds of trifluoromethyl group with cardo group resulted in higher fractional free volume of the polymers. Therefore, the PAs showed low dielectric constant values.23 The lower dielectric constant values of PA ‘B’ and PA ‘A’ might be due to the packing disruptive hexafluoroisopropylidene linkage and tert-butyl groups in comparison to the other polymers. These polymers showed lower dielectric constant value than the analogous bis (phenyl)fluorene based polymers. The fact is attributed to the higher fractional free volume of the bis(phenypheny)fluorene based polymers. The dielectric constant values of these polyamides have been co-related with their gas transport properties [3.3]. Fig. 3 shows WAXD patterns of the polymers (PA ‘A’–‘E’). The broad peaks indicating the amorphous nature of the polymers.
The amorphous nature is due to the bulky cardo fluorene moiety and trifluoro methyl groups which restrict their interchain interaction and free rotation of their molecular structures.2,3,5 Two broad peaks in WAXD patterns have been reported earlier for cardo polyamides, polyimides, polycarbonates and Polyarylates.2,5,24 The WAXS patterns of PAs show a clear shoulder on the high-angle side for all the PAs at similar 2 theta value. The shoulder peak at the larger 2 theta value might be due to the stacking of the aromatic connector group in adjacent polymer chains.5 The peak (halo maxima) observed at lower 2 theta (higher dsp) might be due to loose stacking in polymer matrices exhibited by the enlarging effect cardo bis(phenylphenyl)fluorene moiety.5 It is known that the position of the halo maximum can be considered as an indicator of the most probable intersegmental distance (dsp) between the chains, as calculated from Bragg equation. The decreasing order of dsp values at peak corresponding to lower 2θ value follows the order as PA ‘B’ > PA ‘A’ > PA ‘C’ > PA ‘D’ > PA ‘E’ [Table 1]. Introduction of packing disruptive groups –C(CF3)2 (PA ‘B’) and –CMe3 (PA ‘A’) in acid containing moiety of these two PAs increases the dsp than the other unsubstituted analogs (PA ‘C’, PA ‘D’ and PA ‘E’).23–25
The lower dsp values of PA ‘D’ and PA ‘E’ might be attributed to the more symmetric nature and ordered structures of their backbones. The dsp value can be used as a measure of openness of the polymer chains and it is evident from Tables 1 and 2 that the decreasing order of FFV is nicely matching with decreasing order d-spacing for all the polymers. The PA ‘B’ (hexafluoroisopropylidene group) has higher FFV than PA ‘A’ (–CMe3 group) although both of them have bulky substituent. The above observation attributed to the hexafluoroisopropylidene group is more effective to increase the FFV than tert-butyl group.25 The more trifluoromethyl groups in PA ‘B’ are more effective to reduce cohesive energy density and increase the FFV.5 In general, the bis(phenylphenyl)fluorene based PAs showed lower density, higher FFV and dsp in comparison to their analogous PAs [Table 2].
Two phenyl groups reduces the H-bonding and it is evident from their N–H group density25 values in comparison to the analogous bis(phenyl)fluorene based PAs [Table 2]. It is evident that the openness of the polymer matrix is likely to increase due to the reduced intermolecular hydrogen-bonding.
The TGA thermograms for the polymers are shown in Fig. 4A and B under air and nitrogen respectively. TGA thermograms indicated that all the PAs in the series have high thermal stability with 10% weight loss in the range of 477–517 °C under air and 517–539 °C under nitrogen [Table 3]. The least thermal stability of PA ‘A’ compared to the other PAs in the series is due to presence of easily oxidizable pendant tert-butyl groups.25
 | ||
| Fig. 4 TGA thermograms of the poly(ether amide)s under (A) air and (B) under nitrogen atmosphere (heating rate 10 °C min−1). | ||
The PA ‘B’ also showed 10% weight loss at lower temperature compared to other polymers in these series except PA ‘A’. The added flexibility in PA ‘B’ at hexafluoroisopropylidene group might be responsible for lower degradation temperature at 10% weight loss. The analogous PAs with bis(phenyl)fluorene moiety showed lower thermal stability than these PAs (except PA ‘B’ vs. PA ‘B′’). The above observation might be due to the presence of two thermally stable phenyl groups in bis(phenylphenyl)fluorene moiety. PA ‘C′’ PA ‘D′’ and PA ‘E′’ showed higher thermal stability in air than in nitrogen. Similar observation also found for PAs in some earlier publications.26,27 It is interesting to note that all the PA ‘A’–‘E’ showed almost same Tg values (274–278 °C) and there was no indication of melting or crystallization temperature up to 350 °C in DSC plots (Fig. 5).
Therefore, the acidic counter part of the PAs have no significant role in manipulating the Tg values of the polymers due to the extreme bulky nature of the diamine moiety. In comparison to the analogous poly(ether amide)s, surprisingly these polymers showed lower Tg value (except PA ‘A’ vs. PA ‘A′’). The two substituted pendant phenyl groups in bis(phenylphenyl)fluorene moiety containing PAs reduce N–H group density (H-bonding) and thus favour slight polymer chain motion. Therefore two phenyl groups increase the space between polymer chains as it is evident from their higher FFV values (less cohesive energy density) which resulted in lower Tg compared to the analogous PAs. Wang et al. also reported similar observation for alkyl-substituted cardo poly(aryl ether sulfone)s with unsubstituted analog.28
The PA ‘A’–‘E’ membranes showed tensile strengths of 83–107 MPa, elongations at break of 5.6–8.6% and Young's modulus of 1.74–2.34 Gpa [Table 3]. The highest tensile strength and modulus of PA ‘E’ is attributed to the rigid naphthalene moiety (fused). These PAs showed lower elongation at break in comparison to the analogoues PAs. The difference is attributed to the steric hindrance of the two pendant phenyl rings in bis(phenylphenyl)fluorene moiety.
3.3. Gas transport properties
| Polymer | P (CO2) | P (O2) | P (N2) | P (CH4) | α (CO2/CH4) | α (O2/N2) | α (CO2/N2) | α (CO2/O2) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| a Gas permeability coefficient (P) values taken from ref. 29.b Gas permeability coefficient (P) values taken from ref. 1. P = gas permeability coefficient in barrer. 1 barrer = 10−10 cm3 (STP) cm cm−2 s−1 cm Hg. | |||||||||
| PA ‘A’ | 60.32 | 13.55 | 1.25 | 1.00 | 60.32 | 10.84 | 48.26 | 4.45 | From this study |
| PA ‘B’ | 67.42 | 15.00 | 1.70 | 1.33 | 50.69 | 8.82 | 39.66 | 4.49 | Do |
| PA ‘C’ | 40.38 | 9.05 | 1.11 | 0.93 | 43.42 | 8.15 | 36.38 | 4.46 | Do |
| PA ‘D’ | 38.00 | 7.37 | 0.80 | 0.43 | 88.37 | 9.21 | 47.50 | 5.16 | Do |
| PA ‘E’ | 32.00 | 6.22 | 0.69 | 0.40 | 80.00 | 9.01 | 46.38 | 5.14 | Do |
| PA ‘A′’ | 52.00 | 12.15 | 1.19 | 0.86 | 60.47 | 10.21 | 43.70 | 4.28 | Do |
| PA ‘B′’ | 60.23 | 12.92 | 1.46 | 1.28 | 47.05 | 8.85 | 41.25 | 4.66 | Do |
| PA ‘C′’ | 21.53 | 5.70 | 0.70 | 0.50 | 43.06 | 8.14 | 30.76 | 3.78 | Do |
| PA ‘D′’ | 19.76 | 4.93 | 0.59 | 0.32 | 61.75 | 8.36 | 33.49 | 4.01 | Do |
| PA ‘E′’ | 15.92 | 4.18 | 0.51 | 0.29 | 54.90 | 8.20 | 31.22 | 3.81 | Do |
| FBP/TBIAa | 36.80 | 9.55 | 2.37 | 1.93 | 15.50 | 4.95 | 15.53 | 3.85 | 29 |
| FBP/IAa | 12.40 | 3.03 | 0.62 | 0.57 | 20.10 | 5.32 | 20 | 4.09 | 29 |
| 3gb | 35.30 | 7.35 | 1.36 | 0.91 | 39 | 5.40 | 26 | 4.80 | 1 |
| 3hb | 31.20 | 7.23 | 1.30 | 0.90 | 35 | 5.50 | 24 | 4.32 | 1 |
| Polymer | CO2 | O2 | N2 | CH4 | (CO2/CH4) | (O2/N2) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D | S | D | S | D | S | D | S | αD | αS | αD | αS | |
| PA ‘A’ | 5.17 | 11.67 | 9.49 | 1.43 | 1.95 | 0.64 | 1.82 | 0.55 | 2.84 | 21.22 | 4.87 | 2.23 |
| PA ‘B’ | 9.38 | 7.19 | 11.89 | 1.26 | 2.77 | 0.61 | 2.02 | 0.66 | 4.64 | 10.89 | 4.29 | 2.07 |
| PA ‘C’ | 4.28 | 9.43 | 6.30 | 1.44 | 1.95 | 0.57 | 1.77 | 0.53 | 2.42 | 17.79 | 3.23 | 2.41 |
| PA ‘D’ | 3.08 | 12.34 | 4.93 | 1.50 | 1.34 | 0.60 | 1.75 | 0.25 | 1.76 | 49.36 | 3.68 | 2.50 |
| PA ‘E’ | 2.71 | 11.81 | 3.94 | 1.58 | 1.21 | 0.57 | 1.20 | 0.33 | 2.26 | 35.79 | 3.26 | 2.77 |
| PA ‘A′’ | 4.40 | 11.81 | 5.34 | 2.28 | 1.45 | 0.82 | 1.33 | 0.65 | 3.31 | 18.17 | 3.68 | 2.78 |
| PA ‘B′’ | 5.54 | 10.87 | 5.80 | 2.23 | 1.55 | 0.94 | 1.37 | 0.93 | 4.04 | 11.69 | 3.74 | 2.37 |
| PA ‘C′’ | 2.51 | 8.58 | 3.15 | 1.81 | 1.29 | 0.54 | 1.26 | 0.40 | 1.99 | 21.45 | 2.44 | 3.35 |
| PA ‘D′’ | 2.00 | 9.88 | 2.5 | 1.97 | 1.24 | 0.48 | 1.18 | 0.27 | 1.69 | 36.59 | 2.02 | 4.10 |
| PA ‘E′’ | 1.80 | 8.84 | 1.88 | 2.22 | 1.17 | 0.44 | 1.08 | 0.27 | 1.67 | 32.74 | 1.61 | 5.05 |
The order of fractional free volume of the polymers for all the gases are PA ‘B’ > PA ‘A’ > PA ‘C’ > PA ‘D’ > PA ‘E’. The decreasing order of permeability coefficients of the different gases through these PA membranes is nicely matching with their decreasing order of FFV values of different gases through these polymers. The above decreasing trend of permeability also nicely matches with their decreasing order of dsp [Table 1]. The relation between gas permeability and FFV can be described by the following equation:
| P = Ae(−B/FFV) |
 | ||
| Fig. 6 Correlation of permeability coefficient (P in cm3 (STP) cm cm−2 s−1 cm Hg.) with fractional free volume. | ||
The diffusion coefficients decrease in the order D(O2) > D(CO2) > D(N2) > D(CH4) [Table 5]. For gases like O2, N2, and CH4; the gas diffusivity values decrease with the increase of gas molecule size.28 The diffusivity coefficient value of CO2 was smaller than that of O2 although CO2 has smaller kinetic diameter than O2.
The quadrupolar interaction of CO2 with the amide linkages in the PA backbone reduces its diffusivity coefficient value. Further, CO2 molecule has a “kinetic diameter” of 3.3 Å and its collision diameter of 3.94 Å. Thus, CO2 molecule might have a larger effective size than O2 and as a result D(O2) > D(CO2).32
The decreasing order diffusivity coefficient values of the different gases through these PA membranes is PA ‘B’ > PA ‘A’ > PA ‘C’ > PA ‘D’ > PA ‘E’. The above order actually relates with the openness of the polymeric materials as similar decreasing order also observed for dsp and FFV values for the PAs.
The apparent diffusion coefficients can be governed by the FFV and the free volume size distribution and polymer chain mobility.5 X-ray patterns suggest the difference of the free volume distribution among the cardo polymers.5 The highest gas permeability values for the different gases for PA ‘B’ were in accordance with its highest FFV and dsp values compared to all the other PAs. The difference in the gas permeability coefficient values of the polymers mainly comes from diffusivity coefficient values, rather than the solubility coefficient values of these polymers. Generally, the introduction of hexafluoroisopropylidene group increases gas diffusivity.5 PA ‘A’ and PA ‘B’ showed higher diffusion coefficient values than the less permeable other three polymers. The incorporation of tert-butyl group (PA ‘A’) and hexafluoroisopropylidene linkage (PA ‘B’) in acid containing part increases the diffusivity, FFV as well as permeability coefficient values compared to the other PAs. The higher permeability coefficient values PA ‘A’ and PA ‘B’ are attributed to the reduction in chain packing density (H-bonding) as observed from their higher d-spacing and FFV and lower N–H group density values [Table 2]. PA ‘B’ (hexafluoroisopropylidene group) exhibited higher permeability and lower selectivity for different gas pairs than PA ‘A’ (tert-butyl group). Hexafluoroisopropylidene linkage brings an additional flexibility at the bridged carbon centre of the acidic counterpart of PA ‘B’. Therefore this PA is comparatively non-selective for the size based separation of the penetrant molecules as well as higher –CF3 content in PA ‘B’ compared to the other PAs increases the permeability coefficient for all the gases in a large extent rendering this PA little lower selective. S. C. Kumbharkar et al. also reported lower permselectivity values of polybenzimidazoles based on 4,4′-(hexafluoroisopropylidene)bis(benzoic acid) compared to the 5-tert-butyl-isophthalic acid for CO2/CH4 and O2/N2 gas pairs.25 The PA ‘C’ showed higher permeability than PA ‘D’. The above observation was attributed to the more symmetrical terephthalic acid connector group has high chain packing density as it is observed from its lower FFV and dsp values. Similar results were also found in our previous publications.2,3,33 PA ‘E’ exhibited very low permeability for all the gases due to its higher chain packing density as it is observed from its lowest dsp and lowest FFV [Tables 1 and 2] values. It was in accordance with our previous publication that the compact and rigid naphthalene connector group reduces the permeability.2,33 Ding et al. also observed the lower permeability values for naphthalene acid containing PAs.1 The PA ‘D’ and PA ‘E’ exhibited higher permselectivity values for CO2/CH4 gas pair. The above observation attributed to their more compact structure and these polymers might provide smaller average size free volume elements which are suitable for the diffusion of smaller gas molecules than larger gas molecules.
CO2 showed higher solubility coefficient because of its high critical temperature and it induces some sort of interaction with the carbonyl or amide linkage of the polyamides.2,3 The higher permeability of CO2 for individual polymers resulted from its higher solubility coefficient value. The order of permselectivity for CO2/CH4 gas pair follows as PA ‘D’ > PA ‘E’ > PA ‘A’ > PA ‘B’ > PA ‘C’ and for O2/N2 is PA ‘A’ > PA ‘D’ > PA ‘E’ > PA ‘B’ > PA ‘C’.
According to the “solution–diffusion” mechanism, gas permeability solely depends on gas diffusivity coefficient (D) and solubility coefficient (s). Hence, the contribution of both the terms should be considered for individual gases in order to understand the changes in permeability and permselectivity values of the individual polymers.28 The diffusivity selectivities of uncondensable gas pairs (O2/N2) in the five PAs varied from 3.23 to 4.87, whereas its solubility selectivities were from 2.07 to 2.77. Therefore, the O2/N2 permselectivity values might be governed by the diffusivity selectivity values. Therefore higher permselectivity value for O2/N2 gas pair of PA ‘A’ comes from its higher diffusivity selectivity (4.87) value for this gas pair and lower permselectivity of PA ‘C’ comes from its lower diffusivity selectivity value (3.23) for this gas pair. The gas diffusivity selectivity values of CO2/CH4 gas pair varied from 1.76 to 4.64 and the solubility selectivities were from 10.89 to 49.36. Thus, the solubility selectivity values have an important contribution in CO2/CH4 permselectivity values. Therefore, the overall permselectivity values for CO2/O2, CO2/N2 and CO2/CH4 are mainly due to the solubility selectivities as diffusivity selectivities were comparatively small for these gas pairs [Table 5]. The higher permselectivity for CO2/CH4 gas pair of PA ‘D’ comes from its higher solubility selectivity value for this gas pair. In this scenario PA ‘B’ behave differently.
The dielectric constant values of the PAs varied from 2.20 to 2.65 in 1 MHz at 30 °C (Table 1). The dielectric constant depends on free volume and polarity factor.34 Matsumoto et al. showed a linear relationship between carbon dioxide and methane permeability coefficient values with dielectric constant values for several polyimides.34 They concluded that correlation of dielectric constant with permeability is much more satisfactory than that with fractional free volume. The PAs showed a linear relationship between permeability coefficient values for different gases with their dielectric constant values as shown in Fig. 7.
 | ||
| Fig. 8 Robeson plot35 for a comparison of O2/N2 selectivity vs. O2 permeability coefficients of the PAs under this investigation and some other reported polymers.1,29 | ||
 | ||
| Fig. 9 Robeson plot35 for a comparison of CO2/CH4 selectivity vs. CO2 permeability coefficients of the PAs under this investigation and some other reported polymers.1,29 | ||
In general, these bis(phenylphenyl)fluorene based PAs showed high permeability with higher selectivity than the analogous bis(phenyl)fluorene based polyamides. The higher permeabilty coefficient values of the bis(phenylphenyl)fluorene based PAs might be due to the steric hindrance between two phenyl groups that reduces H-bonding (lower N–H group density), increases the FFV and dsp values compared to the bis(phenyl)fluorene based polyamides [Table 2]. These results are consistent with the higher diffusion coefficient values observed for the PA ‘A’–‘E’ [Table 5]. A. Singh et al. reported that polymers having phenyl substituent were more permeable than their unsubstituted analogs to all gases.36 The higher permselectivity values of PA ‘A’–‘E’ for O2/N2 and CO2/CH4 gas pairs attributed to their higher diffusivity selectivity and solubility selectivity (except PA ‘B’ vs. PA ‘B′’) values respectively in comparison to the analogous PA ‘A′’–‘E′’.
The possible reason of extremely high permeability and selectivity of these new PAs might be due to the increase of chain rigidity and FFV simultaneously.
4. Conclusions
A series of organo soluble new polyamides were prepared from a new diamine monomer, 9,9-bis-[3-phenyl-4-{2′-trifluoromethyl-4′-(4′′-aminophenyl) phenoxy}phenyl]fluorene when reacted with several substituted and non-substituted diacids. The incorporation of bulky bis(phenylphenyl) fluorene moiety, –CF3 group and aryl ether linkage improve the processability, thermal stability, optical and gas permeation properties and reduce dielectric constant values of the polymers. High thermal and mechanical stability of these polymers made them suitable candidates for gas permeation measurements. The gas transport studies revealed that such a designed structure remarkably improved both the gas permeability and permselectivity of these polymers. PA ‘B’ having hexafluoroisopropylidene linkages showed highest permeability in barrer (PCO2 = 67.42 and PO2 = 15) for all the gases whereas PA ‘A’ having tert-butyl moiety exhibited the highest permselectivity towards O2 relative to N2 (10.84) and PA ‘E’ containing naphthalene moiety showed the highest permselectivity towards CO2 over CH4 (88.37) among the series. The comparison of these PAs with analogous bis(phenyl)fluorene PAs revealed that, the phenyl groups in bis(phenylphenyl)fluorene based PAs improves the thermal stability, optical transparency, permeability and permselectivity and lowers the dielectric constant values. All the reported cardo fluorene based PAs showed excellent separation performance for O2/N2 gas pair and their data points exceeded or touched the latest upper boundary limit drawn by Robeson while for CO2/CH4 gas pair the data points laid very close to the upper boundary.Acknowledgements
P. Bandyopadhyay acknowledges CSIR, New Delhi, for providing him a research fellowship to carry out this work. The authors thank AvH Foundation for donation of the GPC instrument used in this work and Department of Science and Technology (DST), India for financial support as project sponsor (Grant no. SR/S3/ME/0008/2010) for this work.References
- Y. Ding and B. Bikson, Polymer, 2002, 43, 4709 CrossRef CAS.
- P. Bandyopadhyay, D. Bera, S. Ghosh and S. Banerjee, J. Membr. Sci., 2013, 417, 413 CrossRef PubMed.
- D. Bera, P. Bandyopadhyay, B. Dasgupta and S. Banerjee, J. Membr. Sci., 2012, 407–408, 116 CrossRef CAS PubMed.
- J. Espeso, A. E. Lozano, J. G. de la Campa and J. de Abajo, J. Membr. Sci., 2006, 280, 659 CrossRef CAS PubMed.
- S. Kazama, T. Teramoto and K. Haraya, J. Membr. Sci., 2002, 207, 91 CrossRef CAS.
- Y. Yampolskii, Macromolecules, 2012, 45, 3298 CrossRef CAS.
- E. Favre, J. Membr. Sci., 2007, 294, 50 CrossRef CAS PubMed.
- Y. Dai, M. D. Guiver, G. P. Robertson, Y. S. Kang, K. J. Lee and J. Y. Jho, Macromolecules, 2004, 37, 1403 CrossRef CAS.
- G. Maier, Angew. Chem., Int. Ed., 1998, 37, 2960 CrossRef.
- S. K. Sen and S. Banerjee, J. Membr. Sci., 2010, 365, 329 CrossRef CAS PubMed.
- B. Dasgupta and S. Banerjee, J. Membr. Sci., 2010, 362, 58 CrossRef CAS PubMed.
- Y. Dai, M. D. Guiver, G. P. Robertson and Y. S. Kang, Macromolecules, 2005, 38, 9670 CrossRef CAS.
- Z. Xu, C. Dannenberg, J. Springer, S. Banerjee and G. Maier, Chem. Mater., 2002, 14, 3271 CrossRef CAS.
- V. Kute and S. Banerjee, J. Appl. Polym. Sci., 2007, 103, 3025 CrossRef CAS.
- M. Redecker, D. D. C. Bradley, M. Inbasekaran, W. W. Wu and E. P. Woo, Adv. Mater., 1999, 11, 241 CrossRef CAS.
- V. Kute and S. Banerjee, Macromol. Chem. Phys., 2003, 204, 2105 CrossRef CAS.
- T. Shimura, M. Watanabe and K. Miyatake, RSC Adv., 2012, 2, 5199 RSC.
- S. Maji and S. Banerjee, J. Appl. Polym. Sci., 2008, 108, 1356 CrossRef CAS.
- S. Maji and S. Banerjee, J. Membr. Sci., 2010, 360, 380 CrossRef CAS PubMed.
- S. Maji and S. Banerjee, J. Membr. Sci., 2010, 349, 145 CrossRef CAS PubMed.
- J. Y. Park and D. R. Paul, J. Membr. Sci., 1997, 125, 23 CrossRef CAS.
- Z. Ge, S. Yang, Z. Tao, J. Liu and L. Fan, Polymer, 2004, 45, 3627 CrossRef CAS PubMed.
- L. Tao, H. Yang, J. Liu, L. Fan and S. Yang, Polymer, 2009, 50, 6009 CrossRef CAS PubMed.
- M. R. Pixton and D. R. Paul, J. Polym. Sci., Part B: Polym. Phys., 1995, 33, 1135 CrossRef CAS.
- S. C. Kumbharkar, P. B. Karadkar and U. K. Kharul, J. Membr. Sci., 2006, 286, 161 CrossRef CAS PubMed.
- H. Yagci and L. J. Mathias, Polymer, 1998, 39, 3779 CrossRef CAS.
- S.-H. Hsiao, C.-P. Yang, M.-H. Chuang and H.-C. Hsiao, J. J. Polym. Sci., Part A: Polym. Chem., 2000, 38, 247 CrossRef CAS.
- Z. Wang, T. Chen and J. Xu, Macromolecules, 2001, 34, 9015 CrossRef CAS.
- M. R. Pixton and D. R. Paul, J. Polym. Sci. Part B: Polym. Physics., 1995, 33, 1135 CrossRef CAS.
- J. S. McHattie, W. J. Koros and D. R. Paul, Polymer, 1992, 33, 1701 CrossRef CAS.
- W. M. Lee, Polym. Eng. Sci., 1980, 20, 65 CAS.
- S. A. Stern, Y. Liu and W. A. Feld, J. Polym. Sci., Part B: Polym. Phys., 1993, 31, 939 CrossRef CAS.
- P. Bandyopadhyay, D. Bera and S. Banerjee, Sep. Purif. Technol., 2013, 104, 138 CrossRef CAS PubMed.
- K. Matsumoto, P. Xu and T. Nishikimi, J. Membr. Sci., 1993, 81, 15 CrossRef CAS.
- L. M. Robeson, J. Membr. Sci., 2008, 320, 390 CrossRef CAS PubMed.
- A. Singh, K. Ghosal, B. D. Freeman, A. E. Lozano, J. G. de la Campa and J. de Abajo, Polymer, 1999, 40, 5715 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |