Nanofluids based on fluorinated graphene oxide for efficient thermal management†
P. M. Sudeepa,
J. Taha-Tijerinab,
P. M. Ajayanb,
T. N. Narayananc and
M. R. Anantharaman*a
aDepartment of Physics, Cochin University of Science and Technology, Kochi-682022, Kerala, India. E-mail: mraiyer@yahoo.com; Tel: +91-484-2577404 ext. 39
bMaterials Science and NanoEngineering Department, Rice University, Houston, TX, USA
cCSIR-Central Electrochemical Research Institute (CSIR-CECRI), Karaikudi-630006, India
First published on 28th May 2014
Abstract
Nanofluids containing various concentrations of graphene oxide (GO), fluorinated graphene oxide (FGO) and highly fluorinated graphene oxide (HFGO) were prepared and their thermal conductivity (TC) was evaluated at different temperatures. Here we report the enhanced TC of stable suspensions of FGO (fluorine (F) ∼5.6%) and HFGO (F ∼ 23%) in comparison to benchmark GO and bare carrier fluids. Enhancement in TC of up to 15–25% is observed in these fluorinated suspensions even at lower filler fractions of 0.01–0.10 wt% and all fluids exhibit filler dependent enhancement in TC. The enhanced TC observed in the case of FGO and HFGO is explained based on liquid layering and percolation mechanism. Moreover, this investigation also unveils the role of F− in enhancing the TC of GO by modifying the surface properties and the effect of liquid layering in determining effective TC of nanofluids.
1. Introduction
Miniaturization of electronic circuitries and development of high pay load electrical/energy instruments demand the successful management of heat energy to a great extent. The discovery of nanofluids, in which solid nanomaterials are dispersed in one of the conventional carrier fluids, leads to the replacement of conventional low efficiency heat transfer fluids with high efficiency colloids. After the first report from Choi et al.,1 numerous nanofluids were reported for their enhanced TC. The dispersants include ceramics, carbon nanostructures, metallic nanoparticles and composites and their thermal efficiency seems to be nominal in lower filler fractions and large amount of dispersants (filler fractions) need to be added to achieve higher TC.2–4 This is a disadvantage for applications, since a large amount nanoparticles will adversely affect the stability of the fluids and thereby result in coagulation of the fluid. Hence researchers are in search for new materials with high TC at low filler fractions in a carrier fluid.Graphene is identified as a highly electrically and thermally conducting (in-plane conductivity) material5 and finds various applications in device fabrication. Recent investigations on graphene also included its functionalised derivatives such as doped graphenes (nitrogen, sulfur, fluorine (F), hydrogen etc.)6,7 and oxidized graphene (GO).8,9 Out of these graphitic structures, GO and fluorinated GO (FGO) received tremendous scientific attention due to their multifunctional properties and applications.10,11 GO and FGO are in plane hybrids containing sp2 and sp3 carbon atoms. Tuning the ratio of sp2 and sp3 domains and incorporation of additional functional groups modifies their physical and chemical properties.7 Fluorine has the highest electronegativity and doping of Fluorine in a graphitic lattice can modify the physical properties of the surface to a large extent. Fluorine binds with carbon in the graphitic lattice by sp3 bonding and hence F− can create additional sp3 domains. FGO has recently been explored in the laboratory of one of the author's and extensively studied for its various properties.7,11 Fluorinated graphene is known for its interesting properties such as high thermal stability, opening of bandgap etc.12 Mickelson et al. studied thermal stability properties of fluorinated carbon nanotubes (F-CNT) and effect of fluorination and defluorination in different carbon nanotubes (CNT) were studied by various groups.13–16 Also fluorination on graphite and its various chemical aspects were studied by Lagow et al.17 Recently we reported fluorinated graphene based primary batteries with enhanced performance.18 Hence the remarkable properties of fluorinated carbon systems are always of interest from a fundamental perspective as well as from an applied angle.
GO has been reported for its thermal properties and it is found that being an amphiphilic material, GO is dispersible in almost all polar and nonpolar liquids and can enhance their TC values.19–22 Brownian motion and percolation mechanism were identified as the main mechanisms for their enhanced TC with temperature and filler addition.23–26 But the role of other adatoms or defects in determining the resultant TC of the material is not understood properly. Fluorine can distort the charge distribution on GO surface and it can bring changes in its physical properties. Recently these authors studied the non-linear optical properties of GO, FGO and highly fluorinated graphene oxide (HFGO) dispersions and observed that FGO can be used as an optical limiter with its optical limiting properties comparable to that of benchmarked C60.27 This study also revealed the role of C–F bonding in providing enhanced non-linear absorption.
Previous report on TC of fluorinated carbon systems rely on phonon–phonon interactions and their investigations suggests a decrease in TC with temperature because of phonon–phonon scattering.28 Moreover reports on the TC of nanofluids based on fluorinated graphene systems are rare in literature or not reported. Thus TC measurements on GO, FGO and HFGO are of interest and can provide information on the role of F− in determining the resultant TC of the materials otherwise having similar morphology and structure. Here we report the TC studies on GO, FGO and HFGO. Temperature dependent TC measurements were conducted using a standard oil bath and filler fraction dependency in TC is also monitored. Since HFGO being highly hydrophobic in nature, due to the large number of C–F bonds on the surface, HFGO was dispersed in N-methylpyrrolidone (NMP). It is found that the TC of FGO in an aqueous medium exhibits enhanced TC while HFGO in NMP also displays large TC values over its base material GO. Enhancement in TC with F− content in graphitic lattice and the mechanism are also discussed.
2. Experimental methods
2.1 Synthesis of GO, FGO and HFGO
GO was prepared by using an “improved method” reported elsewhere.29 For the synthesis of FGO and HFGO, 3 g of fluorinated graphite polymer (Alfa Aesar, 42537) was dispersed in a 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of H2SO4–H3PO4 and stirred at 50 °C for 2 h, 18 g of KMnO4 (Sigma Aldrich) was then added to the mixture in parts.7 The addition of KMnO4 is resulted in an exothermic reaction with an increase in temperature to 90 °C. Upon addition of KMnO4, the color of the solution changed from black to dark brown after which the mixture was subjected to continuous stirring overnight. The mixture was then poured over ice and then 10–12 mL H2O2 added which resulted in effervescence and evolution of yellowish-brown color. Simultaneously, a fluffy dark brown solid phase was observed to precipitate out of solution, at the top of the flask, while the lighter brown phase settled in solution. The two phases were allowed to phase separate overnight. The top phase (HFGO) was simply taken out of solution, while the bottom phase (FGO) was transferred out, then they were washed with 200 mL of water, 200 mL of 30 wt% HCl and 200 mL ethanol. The details of synthesis and its chemical structure are described in our earlier reports.7
1 mixture of H2SO4–H3PO4 and stirred at 50 °C for 2 h, 18 g of KMnO4 (Sigma Aldrich) was then added to the mixture in parts.7 The addition of KMnO4 is resulted in an exothermic reaction with an increase in temperature to 90 °C. Upon addition of KMnO4, the color of the solution changed from black to dark brown after which the mixture was subjected to continuous stirring overnight. The mixture was then poured over ice and then 10–12 mL H2O2 added which resulted in effervescence and evolution of yellowish-brown color. Simultaneously, a fluffy dark brown solid phase was observed to precipitate out of solution, at the top of the flask, while the lighter brown phase settled in solution. The two phases were allowed to phase separate overnight. The top phase (HFGO) was simply taken out of solution, while the bottom phase (FGO) was transferred out, then they were washed with 200 mL of water, 200 mL of 30 wt% HCl and 200 mL ethanol. The details of synthesis and its chemical structure are described in our earlier reports.7
GO and FGO were dispersed in deionized water (DIW) while HFGO was in NMP through extensive ultrasonication for 6 h (Fig. 1). Tyndall scattering was evident when the suspension was illuminated by a red laser beam which is indicative of the colloidal nature of the dispersions. The absorption spectra of GO (Fig. 1b), FGO (Fig. 1c) and HFGO (Fig. 1D) show identical features. Characteristics peaks at 230 nm representing the π → π* transition of C–C, and a shoulder peak ∼300 nm due to n → π* transition of the carbonyl groups are shown in the spectra. Stable suspensions containing various weight percentages of GO, FGO, HFGO (0.01%, 0.02%, 0.05% and 0.10%) were prepared. All the fluids are found to be stable until 2 weeks.
2.2 Characterizations and TC measurements
X-ray diffraction (XRD) pattern (Rigaku, Ultima II) and Raman spectra (Renishaw, 514.6 nm) were recorded to characterize the materials prepared. The size of FGO and HFGO sheets were estimated by Transmission Electron Microscopy (TEM; JEOL-2010). TC was measured using a KS-1 probe (Decagon Device Inc.), which follows a transient hot-wire technique, where a finite length of wire is completely immersed in a finite fluid medium and the wire is electrically heated. While the wire is heated up, the change in resistance is measured as a function of time using a Wheatstone bridge circuitry. The TC value is determined from the heating power and the slope of the temperature change with logarithmic time scale. Precautions were taken to avoid possible errors due to the forced and free convection during the TC measurements.23 The possible errors that can be arising out of free and forced convection mechanisms were reduced by aligning the sensor orientation in the vertical direction with the fluid sample container. It is also ensured that the thermal conduction occurs through direct molecular interactions only, and there is no bulk fluid flow. Though convection of heat transfer occurs when there is bulk fluid flow, it is necessary to isolate all kinds of convection mechanisms that can give rise to an error in TC measurements. The instrument is calibrated with standardized glycerol before each set of measurements. The TC values were verified by repeating the measurements upto 6 times for accuracy. Temperature dependent studies were carried out using an oil bath and in both measurements the same KS1 needle was used.3. Results and discussion
The Raman spectra and XRD pattern of FGO and HFGO are shown in ESI Fig. S1† as it is previously reported.7 Raman spectra exhibited characteristics of D and G peaks at 1322 and 1597 cm−1 respectively with relatively less intensities. The presence of graphitization is clear from Raman spectra. The peaks at 1322 and 1597 cm−1 indicate disorder and order peak respectively arising from graphitic carbon. They resemble the Raman spectrum of GO.4 XRD patterns (ESI Fig. S1†) show the shifting of the characteristic (001) peak of hexagonal graphite at 26° upon functionalisation and exfoliation to ∼10°, resulting in an increase in d-spacing 3.3 to 6 Å and is evident in Fig. S1†. XRD pattern (FGO) follows the same as that of GO.7The TEM images in Fig. S2 (ESI†) illustrate the layered nature of GO, FGO and HFGO. The lateral size of the sheets is found to be in the range of 0.5–1.5 μm and its thickness is few nanometers, indicating the ultra-low thickness (few atomic layers) of the sheets.
3.1 Thermal conductivity studies
The room temperature (296 K) filler fraction dependent enhancement in TC values of GO, FGO and HFGO samples are (with respect to the base fluids) shown in Fig. 2a. Here Keff and K0 represent the thermal conductivities of GO/FGO/HFGO fluid and DIW/NMP, respectively. TC enhancement is calculated as (Keff/K0) − 1. The error bars in the measurements were calculated by the mean deviation from the average 6 data acquisitions and is incorporated. It is evident from the Fig. 2a that in all nanofluids, there is a filler fraction dependent enhancement in the TC values. Out of different fluids, HFGO/NMP shows the highest TC for all filler concentrations. It is known that at lower filler fractions TC follows Maxwell's classical theory but at higher filler fractions it is the percolation process that is predominant.23,30,31 In order to further understand the impact of fluorination on enhancing the TC, TC measurements NMP based GO and HFGO were conducted. Fig. 2b depicts the higher TC values of HFGO in NMP compared to that of GO at the same filler fractions indicate the role of fluorine on enhancing the TC, later it is identified that this enhanced TC values are due to the enhanced liquid layering mechanism of fluorinated materials in polar liquids.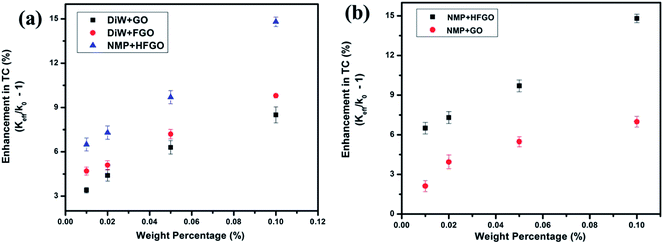 | ||
| Fig. 2 Weight percentage (filler fraction) dependent thermal conductivity variation of various nano fluids: (a) GO/FGO in DiW and HFGO in NMP, (b) GO and HFGO in NMP. | ||
The optical photographs of FGO nanofluid samples at different concentrations after drop casting and drying (slow drying) them over glass plates are shown in Fig. 3 (scale bars represent 10 μm). The images clearly show the enhanced percolation channels with increasing nano filler loading. This indicates that percolation mechanism also plays a crucial role in the effective thermal conductivity of large lateral width (in the present case ∼0.5–1.5 μm FGO/GO) 2D materials based nanofluids.23 The filler fraction dependent enhancement in TC of all samples indicates that, percolation mechanism is the predominant factor in determining the resultant TC of all samples.
Temperature dependent measurements of thermal conductivity of GO, FGO and HFGO is shown in Fig. 4. These measurements were carried out using a thermal bath, and samples were thermally equilibrated for at least 15 minutes before each set of measurements. It is observed that in all these samples TC increases with temperature. In aqueous based nanofluids, FGO exhibits an enhancement of 20% in TC at 0.10 wt% of filler addition with respect to GO. Presence of oxygen containing functional groups in GO will lead to the formation of homogeneous dispersions of GO and FGO in DiW even with mild sonication. Temperature dependent enhancement in TC of all samples indicates the role of Brownian motion in these 2D materials as it is observed in graphene and hexagonal Boron nitride based dispersions.23
Surprisingly, water based dispersion of FGO (FGO–water) shows enhanced TC for all weight fractions when compared to the corresponding GO–water dispersion. Here it is to be noted that GO and FGO are identical in size, structure and morphology. The addition of F− in FGO can modify the local surroundings of the concerned carbon atoms because of its high electronegative nature.32 The Fourier transform infrared (FT-IR) spectrum of GO, FGO and HFGO is shown in Fig. 5. Both spectra of GO and FGO look identical with similar kind of Oxygen functionalities and graphitization. FT-IR spectrum of FGO differs from that of GO only by the presence of C–F bonds at 1208 cm−1. In the case of HFGO, the C–F bond is dominant and other functional groups are less. The amount of F in FGO is estimated to be ∼5.6%4 and it is also found that the ratio of SP2 to SP3 Carbon atoms is similar in both GO and FGO.27 However previous reports suggest that the presence of F in graphitic lattice decreases TC because of enhanced phonon–phonon scattering.28 But in the present study, fluorinated samples show an enhanced TC in all concentrations compared to the pristine samples. It is known that liquid layering and interaction between dispersant and carrier fluid play an important role in determining the effective TC of nanofluids.33 Since F− can induce a charge separation11 in FGO lattice, the interaction between FGO and polar carrier fluids like water or NMP will be higher. This is one of the possible reasons for the enhancement in TC of FGO–water suspensions compared to GO–water. Interestingly HFGO also shows a significant enhancement in TC of ∼26% at 0.10 wt% (where 20% in FGO and 16% in GO at 0.10 wt%). Amount of F content in HFGO is 23%. Hence the effect of F in liquid layer interactions is higher in HFGO–NMP system. This may contribute to an enhanced liquid layering between HFGO flakes and NMP, resulting to an enhanced TC. This study also reveals that phonon–phonon scattering has negligible role in determining the effective TC of the nanofluids.
4. Conclusion
Stable suspensions of GO, FGO in water and HFGO in NMP were prepared and their TC was measured. Temperature dependent enhancement in TC of all samples indicates the roles of Brownian motion and liquid layering in enhancing the TC and percolation effect may contribute to the enhancement in TC upon filler fraction addition. Being a high F-content material with similar morphology, HFGO showed highest TC at any particular temperature and filler fractions compared to GO and FGO.Acknowledgements
P.M.S. acknowledges University Grants Commission (UGC), Govt of India for financial assistance under Research Fellowship in Sciences for Meritorious Students (RFSMS). J.T.T. acknowledges the support from CONACYT (213780). P.M.A. and J.T.T. acknowledge funding from the Army Research Office through MURI program on novel free-Standing 2D crystalline materials focusing on atomic layers of nitrides, oxides, and sulfides (ALNOS) (Award number W911NF-11-1-0362). M.R.A acknowledges Department of Science and Technology (DST), Govt. of India for the financial support through DST-Nanomission project (SR/NM/NS-120/2010(G)). The authors are thankful to Dr Antony George, Rice University for the help in taking optical images.References
- S. U. S. Choi and J. A. Eastman, Enhancing thermal conductivity of fluids with nanoparticles, International mechanical engineering congress and exhibition, San Francisco, CA (United States), 12–17 November 1995 Search PubMed.
- M. B. Bryning, D. E. Milkie, M. F. Islam, J. M. Kikkawa and A. G. Yodh, Thermal conductivity and interfacial resistance in single-wall carbon nanotube epoxy composites, Appl. Phys. Lett., 2005, 87, 161909 CrossRef PubMed.
- M. S. Liu, M. Ching-Cheng Lin, I.-T. Huang and C. C. Wang, Enhancement of thermal conductivity with carbon nanotube for nanofluids, Int. Commun. Heat Mass Transfer, 2005, 32, 1202–1210 CrossRef CAS PubMed.
- J. Vilcakova, R. Moucka, P. Svoboda, M. Ilcikova, N. Kazantseva, M. Hribova, M. Micusik and M. Omastova, Effect of Surfactants and Manufacturing Methods on the Electrical and Thermal Conductivity of Carbon Nanotube/Silicone Composites, Molecules, 2012, 17, 13157–13174 CrossRef CAS PubMed.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang and S. V. Dubonos, et al., Electric field effect in atomically thin carbon films, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- A. L. M. Reddy, A. Srivastava, S. R. Gowda, H. Gullapalli, M. Dubey and P. M. Ajayan, Synthesis of Nitrogen-Doped Graphene Films For Lithium Battery Application, ACS Nano, 2010, 4(11), 6337–6342 CrossRef CAS PubMed.
- A. Mathkar, T. N. Narayanan, L. B. Alemany, P. Cox, P. Nguyen and G. H. Gao, et al., Synthesis of Fluorinated Graphene Oxide and Its Amphiphobic Properties, Part. Part. Syst. Charact., 2013, 30(3), 266–272 CrossRef CAS.
- B. C. Brodie, On the Atomic Weight of Graphite, Philos. Trans. R. Soc. London, 1859, 149, 249–259 CrossRef.
- W. S. Hummers and R. E. Offeman, Preparation of graphitic oxide, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS.
- P. M. Sudeep, T. N. Narayanan, A. Ganesan, M. M. Shaijumon, H. Yang and S. Ozden, et al., Covalently Interconnected Three-Dimensional Graphene Oxide Solids, ACS Nano, 2013, 7(8), 7034–7040 CrossRef CAS PubMed.
- R. Romero-Aburto, T. N. Narayanan, Y. Nagaoka, T. Hasumura, T. M. Mitcham and T. Fukuda, et al., Fluorinated Graphene Oxide; a New Multimodal Material for Biological Applications, Adv. Mater., 2013, 25(39), 5632–5637 CrossRef CAS PubMed.
- R. R. Nair, W. C. Ren, R. Jalil, I. Riaz, V. G. Kravets and L. Britnell, et al., Fluorographene: a Two Dimensional Counterpart of Teflon, Small, 2010, 6(24), 2877–2884 CrossRef CAS PubMed.
- E. T. Mickelson, C. B. Huffman, A. G. Rinzler, R. E. Smalley, R. H. Hauge and J. L. Margrave, Fluorination of single-wall carbon nanotubes, Chem. Phys. Lett., 1998, 296, 188–194 CrossRef CAS.
- E. T. Mickelson, I. W. Chiang, J. L. Zimmerman, P. J. Boul, J. Lozano and J. Liu, et al., Solvation of Fluorinated Single-Wall Carbon Nanotubes in Alcohol Solvents, J. Phys. Chem. B, 1999, 103, 4318–4322 CrossRef CAS.
- J. H. Kang, D. Takhar, O. V. Kuznetsov, V. N. Khabashesku and K. F. Kelly, Fluorination and defluorination of carbon nanotubes: A nanoscale perspective, Chem. Phys. Lett., 2012, 534, 43–47 CrossRef CAS PubMed.
- V. N. Khabashesku, W. E. Billups and J. L. Margrave, Fluorination of Single-Wall Carbon Nanotubes and Subsequent Derivatization Reactions, Acc. Chem. Res., 2002, 35, 1087–1095 CrossRef CAS PubMed.
- R. J. Lagow, R. B. Badachhape, J. L. Wood and J. L. Margrave, Some New Synthetic Approaches to Graphite-Fluorine Chemistry, J. Chem. Soc., Dalton Trans., 1974, 1268–1273 RSC.
- D. Damien, P. M. Sudeep, T. N. Narayanan, M. R. Anantharaman, P. M. Ajayan and M. M. Shaijumon, Fluorinated graphene based electrodes for high performance primary lithium batteries, RSC Adv., 2013, 3, 25702–25706 RSC.
- W. Yu, H. Xie and D. Bao, Enhanced thermal conductivities of nanofluids containing graphene oxide nanosheets, Nanotechnology, 2010, 21, 055705 CrossRef PubMed.
- H. Im and J. Kim, Thermal conductivity of a graphene oxide carbon nanotube hybrid/epoxy composite, Carbon, 2012, 50(15), 5429–5440 CrossRef CAS PubMed.
- B. Wang, J. Hao and H. Li, Remarkable improvements in the stability and thermal conductivity of graphite/ethylene glycol nanofluids caused by a graphene oxide percolation structure, Dalton Trans., 2013, 42, 5866–5873 RSC.
- S. S. Gupta, V. Manoj Siva, S. Krishnan, T. S. Sreeprasad, P. K. Singh, T. Pradeep and S. K. Das, Thermal conductivity enhancement of nanofluids containing graphene nanosheets, J. Appl. Phys., 2011, 110, 084302 CrossRef PubMed.
- J. T. Tijerina, T. N. Narayanan, G. Gao, M. Rohde, D. A. Tsentalovich and M. Pasquali, et al., Electrically Insulating Thermal Nano-Oils Using 2D Fillers, ACS Nano, 2012, 6(2), 1214–1220 CrossRef PubMed.
- J. Taha-Tijerina, T. N. Narayanan, C. S. Tiwary, K. Lozano, M. Chipara and P. M. Ajayan, Nanodiamond Based Thermal Fluids, ACS Appl. Mater. Interfaces, 2014, 6(7), 4778–4785 CAS.
- J. Taha-Tijerina, L. Peña-Paras, T. N. Narayanan, L. Garza, D. Maldonado and P. M. Ajayan, et al., Wear, 2013, 302(1–2), 1241–1248 CrossRef CAS PubMed.
- C. Kleinstreuer and Y. Feng, Experimental and theoretical studies of nanofluid thermal conductivity enhancement: a review, Nanoscale Res. Lett., 2011, 6(1), 229 CrossRef PubMed.
- P. Chantharasupawong, R. Philip, T. N. Narayanan, P. M. Sudeep, A. Mathkar and P. M. Ajayan, et al., Optical Power Limiting in Fluorinated Graphene Oxide: An Insight into the Nonlinear Optical Properties, J. Phys. Chem. C, 2012, 116, 25955–25961 CAS.
- F. Gardea, Electrical and thermal experimental characterization and modeling of carbon nanotube/epoxy composites, M.Sc thesis, Texas A&M University, 2011.
- D. C. Marcano, D. V. Kosynkin, J. M. Berlin, A. Sinitskii, Z. Sun and A. Slesarev, et al., Improved Synthesis of Graphene Oxide, ACS Nano, 2010, 4, 4806–4814 CrossRef CAS PubMed.
- J. Buongiorno, D. C. Venerus, N. Prabhat, T. McKrell, J. Townsend and R. Christianson, et al., A benchmark study on the thermal conductivity of nanofluids, J. Appl. Phys., 2009, 106, 094312 CrossRef PubMed.
- V. Trisaksria and S. Wongwises, Critical review of heat transfer characteristics of nanofluids, Renewable Sustainable Energy Rev., 2007, 11, 512–523 CrossRef PubMed.
- K. N. Kudin, H. F. Bettinger and G. E. Scuseria, Fluorinated single-wall carbon nanotubes, Phys. Rev. B: Condens. Matter Mater. Phys., 2001, 63, 045413 CrossRef.
- W. Yu and S. U. S. Choi, The role of interfacial layers in the enhanced thermal conductivity of nanofluids: A renovated Maxwell model, J. Nanopart. Res., 2003, 5, 167–171 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra00843j |
| This journal is © The Royal Society of Chemistry 2014 |




