Microwave irradiated synthesis of 2-bromo(chloro)indoles via intramolecular cyclization of 2-(gem-dibromo(chloro)vinyl)anilines in the presence of TBAF under metal-free conditions†
Min Wanga,
Pinhua Liab,
Wei Chena and
Lei Wang*ac
aDepartment of Chemistry, Huaibei Normal University, Huaibei, Anhui 235000, P.R. China. E-mail: leiwang@chnu.edu.cn; Fax: +86 561-309-0518; Tel: +86 561-380-2069
bKey Laboratory of Synthetic Chemistry of Natural Substances, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai 200032, P.R. China
cState Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai, 200032, P.R. China
First published on 4th June 2014
Abstract
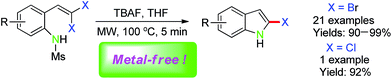
2-Bromo(chloro)indoles were readily and efficiently prepared through TBAF-promoted intramolecular cyclization of 2-(gem-dibromo(chloro)vinyl)anilines in excellent yields under metal-free and microwave irradiation conditions.
Introduction
The indole skeleton is one of the important structural motifs widely found in dyes, natural products, materials, and pharmaceutically active compounds, such as antibiotic, anticancer and anti HIV agents.1 In addition, the indole derivatives are also used as starting materials for the synthesis of a large number of alkaloids. Many halogenated indole derivatives are also found in nature, and several brominated indole natural products have been isolated.2 They are also present in biologically active compounds, such as Convolutindole A,2d and polybrominated bisindoles.2e,fDue to the electron-rich nature of indoles, the preparation of brominated indoles is still a challenge. The most straightforward method is electrophilic bromination of indoles, and 3-bromoindoles are obtained, along with the by-products.3 While unprotected 2-bromoindoles are useful for numerous applications,4 their preparation is often difficult. Although the synthesis of 2-bromoindole based on lithiation of unsubstituted indole, followed by bromination was developed, the extension of its derivatives was less investigated owing to the limitation of regioselectivity and functional group tolerance on lithiation.5
gem-Dihaloolefins, as one of the important synthetic intermediates for their higher reactivity and easy availability have been attracted much attention in recent years.6 Especially, the construction of various functionalized indole derivatives from 2-(2,2-dibromovinyl)anilines and 2-(2,2-dibromovinyl)(N-substituted)anilines through the transition-metal-catalyzed cross-coupling methodologies, such as carbon–nitrogen/carbon–carbon,7 carbon–nitrogen/carbon–nitrogen,8 carbon–nitrogen/carbon–phosphine,7a carbon–nitrogen/carbon–hydrogen,9 carbon–nitrogen/carbonylation,10 and carbon–nitrogen/carbonylation/carbon–carbon tandem reactions11 has been reported. Recently, Lautens et al. developed an efficient method for the synthesis of 2-bromoindoles via Pd/Pt-Bu3-catalyzed intramolecular reactions of 2-(gem-dibromovinyl)anilines.12
Microwave heating is an attractive tool that has recently been used in organic synthesis. And it presents a powerful and green alternative to conventional synthesis, which helps the chemical process more economic and soft.13 In continuing our efforts on the organic transformations of gem-dihaloolefins under metal-free conditions14a–c and the solid-phase construction of indole derivatives based on a traceless, activating sulfonyl linker by Zhang,14d herein we developed a tetra-butylammonium fluoride (TBAF)-promoted intramolecular cyclization of 2-(gem-dibromovinyl)-N-methylsulfonylanilines15 for the synthesis of 2-bromoindoles under microwave irradiation conditions. The reactions generated unprotected 2-bromoindoles under metal-free and mild reaction conditions in nearly quantitatively yields. In addition, this methodology can also be extended to synthesis of 2-chloroindoles (Scheme 1).
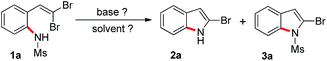
In our initial attempt to obtain 2-bromoindole from the corresponding 2-(gem-dibromovinyl)aniline in the presence of TBAF in THF at 80 °C for 8 h, a complicated mixture of compounds was obtained, but no desired 2-bromoindole was observed. When N-substituted derivatives of 2-(gem-dibromovinyl)aniline, such as N-benzyl, N-acetyl, and N-trifluoroacetyl were used as substrates, however, no desired 2-bromoindole was detected and a complicated mixture of compounds was generated. While 2-(gem-dibromovinyl)-N-tert-butoxycarbonylaniline was used, 2-(bromoethynyl)-N-tert-butoxycarbonylaniline was isolated in 91% yield. To our delight, 94% yield of 2-bromoindole was obtained when 2-(gem-dibromovinyl)-N-methylsulfonylaniline was used as substrate. However, 2-(gem-dibromovinyl)-N-(p-tolylsulfonyl)aniline was inferior and generated 2-bromoindole in 76% yield. The results indicated that this cyclization depends on the nitrogen substituents of substrates. When the amine is activated by a strong electron-withdrawing group such as sulfonyl, the tandem reaction can occur efficiently in one pot. The N-sulfonyl linker serves as a dual-activating group to undergo 2-bromoindoles formation. After first-step cyclization, the sulfonamide linkage proceeds nitrogen–sulfur bond cleavage to remove sulfonyl group.
To further optimize the reaction conditions for the synthesis of 2-bromoindole (2a) from 2-(gem-dibromovinyl)-N-methylsulfonylaniline (1a) through a base-promoted intramolecular cyclization, a variety of bases were examined. The results were summarized in Table 1. As the analogues of TBAF, TBAC (tetra-butylammonium chloride), TBAB (tetra-butylammonium bromide), TBAI (tetra-butylammonium iodide), or TBA·HSO4 (tetra-butylammonium hydrogen sulfate) was used instead of TBAF, no product was detected and starting material was unchanged and recovered (Table 1, entries 2−5). Meanwhile, TBA·OH (tetra-butylammonium hydroxide) and TBA·OAc (tetra-butylammonium acetate) generated 2-bromo-N-methylsulfonylindole (3a) in 35 and 72% yields, and no 2a was obtained (Table 1, entries 6 and 7). Other bases, KF, CsF, Et3N, and DABCO were ineffective promoters to the reaction (Table 1, entries 8−11). However, K2CO3, K3PO4, and tBuOK gave 3a or a mixture of 2a and 3a in inferior yields (Table 1, entries 12−14). With respect to TBAF loading, 2 equiv. of TBAF was found to be optimal. On the other hand, the influence of solvent on the reaction was also examined, and the results indicated that THF was the best one among the solvents tested. Other solvents, DMF, CH3CN, H2O, DMSO, dioxane, toluene, and C2H5OH were inferior (Table 1, entries 15−21). However, only 43% yield of 2a was obtained in the absence of solvent (Table 1, entry 22). The further investigation indicated that the reaction was completed at 80 °C in 8 h. Gratifyingly, the reaction was completed under microwave irradiation conditions at 100 W and 100 °C in 5 min and 99% yield of 2a was obtained (Table 1, entries 23−26).
| Entry | Base | Solvent | Yieldb [%] | |
|---|---|---|---|---|
| 2a | 3a | |||
| a Reaction conditions: 2-(gem-dibromovinyl)-N-methylsulfonylaniline (1a, 0.50 mmol), base (1.0 mmol), solvent (2.0 mL) at 80 °C for 8 h.b Isolated yields.c The reaction was irradiated at 100 W and 80 °C for 20 min.d The reaction was irradiated at 100 W and 100 °C for 20 min.e The reaction was irradiated at 100 W and 100 °C for 5 min.f The reaction was irradiated at 100 W and 100 °C for 2 min.g Pd(OAc) (5.0 ppm in THF) was added.h Pd(OAc) (10.0 ppm in THF) was added.i CuI (50.0 ppm in THF) was added. | ||||
| 1 | TBAF | THF | 94 | 0 |
| 2 | TBAC | THF | 0 | 0 |
| 3 | TBAB | THF | 0 | 0 |
| 4 | TBAI | THF | 0 | 0 |
| 5 | TBA·HSO4 | THF | 0 | 0 |
| 6 | TBA·OH | THF | 0 | 35 |
| 7 | TBA·OAc | THF | 0 | 72 |
| 8 | KF | THF | 0 | 0 |
| 9 | CsF | THF | 0 | 0 |
| 10 | Et3N | THF | 0 | 0 |
| 11 | DABCO | THF | 0 | 0 |
| 12 | K2CO3 | THF | 0 | 18 |
| 13 | K3PO4 | THF | 22 | 27 |
| 14 | tBuOK | THF | 36 | 51 |
| 15 | TBAF | DMF | 91 | Trace |
| 16 | TBAF | CH3CN | 78 | Trace |
| 17 | TBAF | H2O | 67 | Trace |
| 18 | TBAF | DMSO | 52 | Trace |
| 19 | TBAF | Dioxane | 27 | 16 |
| 20 | TBAF | Toluene | 25 | 0 |
| 21 | TBAF | C2H5OH | 19 | 23 |
| 22 | TBAF | — | 43 | Trace |
| 23c | TBAF | THF | 57 | 10 |
| 24d | TBAF | THF | 99 | 0 |
| 25e | TBAF | THF | 99 | 0 |
| 26f | TBAF | THF | 72 | 0 |
| 27g | — | THF | <5 | — |
| 28h | — | THF | <5 | — |
| 29i | — | THF | <5 | — |
Organic reactions performed in the absence of transition metal have received much attention, because they can overcome the drawbacks of the expensive, poisonous, and air-sensitive properties of metals or organometallics, and metal-free reaction is one of the best choices in the pharmaceutical industry due to the avoidance of metal contamination.16 However, some reactions turned out to be actually catalyzed by a trace amount of Pd or Cu contamination, not really “transition-metal-free”.17 In order to figure out whether the model reaction completed in the presence of trace amount of transition metal or not, additional trace amount of Pd (5.0–10.0 ppm) or Cu (50.0 ppm) was added to the reaction of 1a in THF under microwave irradiation condition without TBAF, providing less than 5% yield of 2a (Table 1, entries 27−29).14c,17e,f This result indicated that TBAF plays an important role in the intramolecular cyclization reaction of 1a.
On the basis of the optimized reaction conditions (in the presence of TBAF under microwave irradiation at 100 W and 100 °C for 5 min), the scope of this transformation was investigated and the results were summarized in Table 2. A variety of 2-(gem-dibromovinyl)-N-methylsulfonylanilines bearing substituents on the benzene rings were examined. The results showed that a number of functional groups, including electron-withdrawing and electron-donating ones were tolerated, and the corresponding 2-bromoindoles were generated in excellent yields (Table 2, entries 1−21). Halogen substituents, such as F, Cl, Br and I, on the 4-, 5-, or 3-position of substrates (1b–j) underwent the intramolecular cyclization very smoothly and afforded the excellent yields of the corresponding di-halogenated indoles 2b–j (Table 2, entries 2−10), which could provide the further transformation via transition-metal-catalyzed cross-coupling reactions. 2-(gem-Dibromovinyl)-N-methylsulfonylanilines with an electron-donating functionality, such as CH3, CH3O, C6H5CH2O, CH3SO2O, p-CH3OC6H4 or OCH2O on the anilines also underwent the cyclization very well to generate the corresponding products 2k–q in 90–96% yields (Table 2, entries 11−17). It should be noted that the reaction could tolerate ortho-substituted group (2q). Under the present reaction conditions, 1-(gem-dibromovinyl)-2-naphthylamine (1r) also underwent the reaction very well to generate the desired product 2r in 96% yield. Substrates (1s–u) with electron-withdrawing groups, CH3OCO, and CH3CO on the 4-, and 3-position of anilines also gave the corresponding products 2s–u in 90–93% yields. More remarkable observation was that 2-(gem-dichlorovinyl)-N-methylsulfonylaniline (1v) also could proceed the reaction to generate 2-chloroindole (2v) with high yield in DMF at 110 °C under microwave irradiation (Table 2, entry 22). It is important to note that the reaction scale was increased up to 10 mmol under TBAF/THF conditions for 10 min, 94% isolated yield of 2a was obtained from 1a (Scheme 2).
| Entry | 1, R | X | 2 | Yieldb [%] |
|---|---|---|---|---|
| a Reaction conditions: 1 (0.50 mmol), TBAF (1.0 mmol), THF (2.0 mL) and the reaction mixture was irradiated at 100 W and 100 °C for 5 min.b Isolated yields.c DMF (2.0 mL), the reaction mixture was irradiated at 100 W and 110 °C for 10 min. | ||||
| 1 | 1a, H | Br | 2a | 99 |
| 2 | 1b, 4-F | Br | 2b | 97 |
| 3 | 1c, 4-Cl | Br | 2c | 96 |
| 4 | 1d, 4-Br | Br | 2d | 97 |
| 5 | 1e, 4-I | Br | 2e | 94 |
| 6 | 1f, 5-F | Br | 2f | 91 |
| 7 | 1g, 5-Cl | Br | 2g | 93 |
| 8 | 1h, 5-Br | Br | 2h | 91 |
| 9 | 1i, 3-F | Br | 2i | 92 |
| 10 | 1j, 3-Cl | Br | 2j | 91 |
| 11 | 1k, 4-CH3 | Br | 2k | 95 |
| 12 | 1l, 4-CH3O | Br | 2l | 93 |
| 13 | 1m, 4-C6H4CH2O | Br | 2m | 92 |
| 14 | 1n, 4-CH3SO2O | Br | 2n | 94 |
| 15 | 1o, 4-(p-CH3O)C6H4 | Br | 2o | 96 |
| 16 | 1p, 4,5-(OCH2O) | Br | 2p | 91 |
| 17 | 1q, 6-CH3 | Br | 2q | 90 |
| 18 | 1r, 5,6-benzo | Br | 2r | 96 |
| 19 | 1s, 4-CH3OCO | Br | 2s | 91 |
| 20 | 1t, 5-CH3OCO | Br | 2t | 93 |
| 21 | 1u, 5-CH3CO | Br | 2u | 90 |
| 22 | 1v, H | Cl | 2v | 91c |
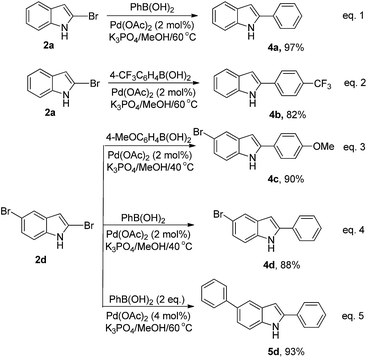
2-Arylindoles exhibit high biological activities, such as antiestrogen, h5-HT2A antagonism, anti-inflammatory, and cytotoxicity.18 Palladium-catalyzed cross-coupling of 2-haloindoles with an arylmetal species is one of the useful strategies for the synthesis of 2-arylindoles,19 but this application has been limited by the inaccessibility of the 2-haloindoles.20 With the prepared 2-bromoindoles in our hands, transformation of them into the corresponding 2-arylindoles was investigated via palladium-catalyzed cross-coupling with arylboronic acids (Scheme 3). The results indicated that the corresponding products were obtained in high yields under the Suzuki reaction conditions (Scheme 3, eqn (1) and (2)). In the analogy studies, a special ligand such as dppf, S-Phos, or P(t-Bu)3 is essential.7a,b,e Futhermore, only 4c, 4d or 5d was obtained in high yield during the reaction of 2d with 4-MeOC6H4B(OH)2 or PhB(OH)2 by controlling Suzuki coupling conditions (Scheme 3, eqn (3)–(5)).
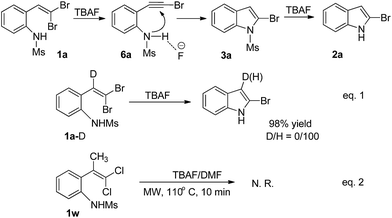
A reaction mechanism for the generation of 2-bromoindole (2a) was proposed in Scheme 4. Initially, an elimination of HBr from 1a to intermediate 6a proceeded smoothly in the presence of TBAF,14a,b followed by an intramolecular nucleophilic addition of nitrogen to carbon–carbon triple bond of 6a to afford a cyclization product 3a with the assistance of fluoride anion. Finally, the obtained 3a underwent a cleavage of the sulfonamide linkage to afford the desired product 2a under TBAF conditions.14d,21
To verify the formation of intermediates 6a and 3a in the reaction, control experiments of 1a and its isotope investigation were conducted. When 1a was carried out in the presence of TBAF (1.0 equiv.) under microwave irradiation at 100 W and 100 °C for 5 min, 3a was isolated in 61% yield, and 2a was obtained in 15% yield. On the other hand, when deuterium-labeled 1a-D was carried out under the present reaction conditions, 2a was obtained in 98% yield, and no 2-bromo-3-D-indole was detected (Scheme 4, eqn (1)). It also supports that the reaction was through an alkynyl bromide intermediate 6a. We also tried the reaction of 2-(1′-methyl-2′,2′-dichlorovinyl)-N-methylsulfonylaniline (1w) in the presence of TBAF in DMF under present reaction conditions, however, no product was isolated and 1w was recovered in 98% yield (Scheme 4, eqn (2)). In addition, ICP analysis indicated that Cu and Pd in the reaction mixture were 0.27 and 0.03 ppm, respectively.22
In summary, we have developed an efficient and facile TBAF-promoted intramolecular cyclization of 2-(gem-dibromo(chloro)vinyl)anilines for the synthesis of 2-bromo(chloro)indoles under microwave irradiation conditions. The reactions were carried out in THF in the absence of metal, and generated the corresponding products in excellent yields. It is important to note that when the amine is activated by a strong electron-withdrawing group such as sulfonyl, the tandem reaction can occur efficiently in one pot. The reaction mechanism investigation indicated that the sulfonyl linker serves as a dual-activating group to undergo indole cyclization. And after indole formation, it is activated and subsequently proceeds nitrogen–sulfur bond cleavage to remove sulfonyl group promoted by TBAF. Moreover, further transformation of 2-bromoindoles via palladium-catalyzed cross-coupling can be carried out.
Experimental section
General remarks
All reagents were purchased from commercial suppliers and used without further purification. 2-(gem-Dibromovinyl)-N-methylsulfonylanilines were prepared according to the literature.7b All TBAF (tetra-n-butylammonium fluoride)-promoted intramolecular cyclization reactions of gem-dibromoolefins were carried out under microwave irradiation conditions and air atmosphere. 1H NMR and 13C NMR spectra were measured on a Bruker Avance NMR spectrometer (400 MHz or 100 MHz, respectively) with CDCl3 as solvent and recorded in ppm relative to internal tetramethylsilane standard. The peak patterns are indicated as follows: s, singlet; d, doublet; t, triplet; m, multiplet; q, quartet. The coupling constants, J, are reported in Hertz (Hz). High resolution mass spectroscopy data of the product were collected on a Waters Micromass GCT instrument. All MW reactions were carried out in a Discover SP (CEM) microwave reactor.Typical procedure for the synthesis of 2-bromoindole (2a) via microwave irradiated intramolecular cyclization of 2-(gem-dibromovinyl)-N-methylsulfonylaniline (1a) in the presence of TBAF
In a 10 mL of sealable reaction tube with a Teflon-coated screw cap equipped with a magnetic stir bar was charged with 2-(gem-dibromovinyl)-N-methylsulfonylaniline (1a, 0.50 mmol), TBAF (THF solution, 1.0 mol L−1, 1.0 mL, 1.0 mmol) and THF (2.0 mL). The reaction vessel was placed in a Discover SP (CEM) microwave reactor, and the reaction mixture was irradiated at 100 W and 100 °C for 5 min. Then it was cooled to room temperature, extracted twice with Et2O. The organic layers were combined, dried over Na2SO4, and concentrated under reduced pressure to yield the crude product, which was further purified by flash chromatography on silica gel (eluant: hexane/ethyl acetate) to give the desired product 2-bromoindole (2a).Acknowledgements
We gratefully acknowledge financial support by the National Natural Science Foundation of China (no. 20372095, 21172092, and 21002039), the Project of Science and Technology of the Department of Education, Anhui (no. KJ2010ZD09, KJ2012A253).Notes and references
- For selected reviews, see: (a) S. Cacchi and G. Fabrizi, Chem. Rev., 2005, 105, 2873 CrossRef CAS PubMed; (b) G. R. Humphrey and J. T. Kuethe, Chem. Rev., 2006, 106, 2875 CrossRef CAS PubMed; (c) M. Somei and F. Yamada, Nat. Prod. Rep., 2004, 21, 278 RSC; (d) M. Somei and F. Yamada, Nat. Prod. Rep., 2005, 22, 73 RSC; (e) For selected examples, see: J. F. Payack, E. Vazquez, L. Matty, M. H. Kress and J. McNamara, J. Org. Chem., 2005, 70, 175 CrossRef CAS PubMed; (f) N. K. Garg, D. D. Caspi and B. M. Stoltz, J. Am. Chem. Soc., 2005, 127, 5970 CrossRef CAS PubMed.
- For selected reviews, see: (a) R. J. Sundberg, Indoles, Academic Press, San Diego, 1996 Search PubMed; (b) G. W. J. Gribble, J. Chem. Soc., Perkin Trans. 1, 2000, 1045 RSC; (c) G. W. J. Gribble, Nat. Prod., 1992, 55, 1353 CrossRef CAS; (d) C. K. Narkowicz, A. J. Blackman, E. Lacey, J. H. Gill and K. J. Heiland, Nat. Prod., 2002, 65, 938 CrossRef CAS PubMed; (e) For selected examples, see: R. S. Norton and R. J. Wells, J. Am. Chem. Soc., 1982, 104, 3628 CrossRef CAS; (f) G. Bringmann, S. Tasler, H. Endress, J. Kraus, K. Messer, M. Wohlfarth and W. Lobin, J. Am. Chem. Soc., 2001, 123, 2703 CrossRef CAS PubMed.
- (a) J. C. Powers, in The Chemistry of Heterocyclic Compounds, ed. W. J. Houlihan, Wiley and Sons, New York, 1972, vol. 25, part 2, p. 128 Search PubMed; (b) W. C. Frank, Y. C. Kim and R. F. Heck, J. Org. Chem., 1978, 43, 2947 CrossRef CAS.
- (a) M. S. Techenor, J. D. Trzupek, D. B. Kastrinsky, F. Shiga, I. Hwang and D. L. Boger, J. Am. Chem. Soc., 2006, 128, 15683 CrossRef PubMed; (b) J. M. Herbert, Tetrahedron Lett., 2004, 45, 817 CrossRef CAS PubMed; (c) M. G. Bursavicha, N. Brooijmans, L. Feldberg, I. Hollander, S. Kim, S. Lombardi, K. Park, R. Mallon and A. M. Gilbert, Bioorg. Med. Chem. Lett., 2010, 20, 2586 CrossRef PubMed; (d) K. Dinnell, G. G. Chicchi, M. J. Dhar, J. M. Elliott, G. J. Hollingworth, M. M. Kurtz, M. P. Ridgill, W. Rycroft, K.-L. Tsao, A. R. Williams and C. J. Swaina, Bioorg. Med. Chem. Lett., 2001, 11, 1237 CrossRef CAS; (e) Y. Liu and G. W. Gribble, Tetrahedron Lett., 2000, 41, 8717 CrossRef CAS.
- (a) J. Bergman and L. Venemalm, J. Org. Chem., 1992, 57, 2495 CrossRef CAS; (b) For the preparation of indoles regarding the cyclisations of alkynylanilines, see: H.-C. Zhang, K. K. Brumfield and B. E. Maryanoff, Tetrahedron Lett., 1997, 38, 2439 CrossRef CAS; (c) R. C. Larock and E. K. Yum, J. Am. Chem. Soc., 1991, 113, 6689 CrossRef CAS; (d) H.-C. Zhang, K. K. Brumfield, L. Jaroskova and B. E. Maryanoff, Tetrahedron Lett., 1998, 39, 4449 CrossRef CAS; (e) M. D. Collini and J. W. Ellingboe, Tetrahedron Lett., 1997, 38, 7963 CrossRef CAS; (f) M. C. Fagnola, I. Candiani, G. Visentin, W. Cabri, F. Zarini, N. Mongelli and A. Bedeschi, Tetrahedron Lett., 1997, 38, 2307 CrossRef CAS; (g) T. Sakamoto, Y. Kondo and H. Yamanaka, Heterocycles, 1988, 27, 2225 CrossRef CAS PubMed; (h) A. Arcadi, S. Cacchi and F. Marinelli, Tetrahedron Lett., 1992, 33, 3915 CrossRef CAS; (i) M. C. Yasuhara, Y. Kanamori, M. Kaeko, A. Numata, Y. Kondo and T. Sakamoto, J. Chem. Soc., Perkin Trans. 1, 1999, 529 RSC.
- For recent reviews, see: (a) G. Chelucci, Chem. Rev., 2012, 112, 1344 CrossRef CAS PubMed; (b) S. Cacchi, G. Fabrizi and A. Goggiamani, Org. Biomol. Chem., 2011, 9, 641 RSC; (c) F. Legrand, K. Jouvin and G. Evano, Isr. J. Chem., 2010, 50, 588 CrossRef CAS; (d) J.-R. Wang and K. Manabe, Synthesis, 2009, 1405 Search PubMed; (e) Handbook of Organopalladium Chemistry for Organic Synthesis, ed. Negishi, Wiley-Interscience, New York, 2002, p. 650 Search PubMed.
- (a) S. Thielges, E. Meddah, P. Bisseret and J. Eustache, Tetrahedron Lett., 2004, 45, 907 CrossRef CAS PubMed; (b) Y.-Q. Fang and M. Lautens, J. Org. Chem., 2008, 73, 538 CrossRef CAS PubMed; (c) Y.-Q. Fang and M. Lautens, Org. Lett., 2005, 7, 3549 CrossRef CAS PubMed; (d) A. Fayol, Y.-Q. Fang and M. Lautens, Org. Lett., 2006, 8, 4203 CrossRef CAS PubMed; (e) M. Nagamochi, Y.-Q. Fang and M. Lautens, Org. Lett., 2007, 9, 2955 CrossRef CAS PubMed; (f) Y.-Q. Fang, J. Yuen and M. Lautens, J. Org. Chem., 2007, 72, 5152 CrossRef CAS PubMed; (g) Y.-Q. Fang, R. Karisch and M. Lautens, J. Org. Chem., 2007, 72, 1341 CrossRef CAS PubMed.
- J. Yuen, Y.-Q. Fang and M. Lautens, Org. Lett., 2006, 8, 653 CrossRef CAS PubMed.
- (a) C. S. Bryan and M. Lautens, Org. Lett., 2008, 10, 4633 CrossRef CAS PubMed; (b) Z.-J. Wang, J.-G. Yang, F. Yang and W. Bao, Org. Lett., 2010, 12, 3034 CrossRef CAS PubMed; (c) Z.-J. Wang, F. Yang, X. Lv and W. Bao, J. Org. Chem., 2011, 76, 967 CrossRef CAS PubMed; (d) X.-R. Qin, X.-F. Cong, D.-B. Zhao, J.-S. You and J.-B. Lan, Chem. Commun., 2011, 47, 5611 RSC; (e) W. Chen, M. Wang, P. Li and L. Wang, Tetrahedron, 2011, 67, 5913 CrossRef CAS PubMed.
- T. O. Vieira, L. A. Meaney, Y.-L. Shi and H. Alper, Org. Lett., 2008, 10, 4899 CrossRef CAS PubMed.
- M. Arthuis, R. Pontikis and J.-C. Florent, Org. Lett., 2009, 11, 4608 CrossRef CAS PubMed.
- (a) S. G. Newman, V. Aureggi, C. S. Bryan and M. Lautens, Chem. Commun., 2009, 5236 RSC; (b) S. G. Newman and M. Lautens, J. Am. Chem. Soc., 2010, 132, 11416 CrossRef CAS PubMed.
- (a) C. O. Kappe and A. Stadler, Microwaves in Organic and Medicinal Chemistry, Wiley-VCH, Weinheim, 2005 Search PubMed; (b) A. Loupy, Microwaves in Organic Synthesis, Wiley-VCH, Weinheim, 2nd edn, 2006 Search PubMed; (c) C. O. Kappe, D. Dallinger and S. S. Murphree, Practical Microwave Synthesis for Organic Chemists: Strategies, Instruments, and Protocols, Wiley-VCH, Weinheim, 2009 Search PubMed; (d) J. D. Moseley and C. O. Kappe, Green Chem., 2011, 13, 794 RSC.
- (a) W. Chen, Y. Zhang, L. Zhang, M. Wang and L. Wang, Chem. Commun., 2011, 47, 10476 RSC; (b) M. Okutani and Y. Mori, J. Org. Chem., 2009, 74, 442 CrossRef CAS PubMed; (c) P. Li, Y. Ji, W. Chen, X. Zhang and L. Wang, RSC Adv., 2013, 3, 73 RSC; (d) H.-C. Zhang, H. Ye, A. F. Moretto, K. K. Brumfield and B. E. Maryanoff, Org. Lett., 2000, 2, 89 CrossRef CAS PubMed.
- B. Jiang, K. Tao, W. Shen and J. Zhang, Tetrahedron Lett., 2010, 50, 6342 CrossRef PubMed.
- (a) C.-L. Sun, H. Li, D.-G. Yu, M. Yu, X. Zhou, X.-Y. Lu, K. Huang, S.-F. Zheng, B.-J. Li and Z.-J. Shi, Nat. Chem., 2010, 2, 1044 CrossRef CAS PubMed; (b) E. Shirakawa, K. Itoh, T. Higashino and T. Hayashi, J. Am. Chem. Soc., 2010, 132, 15537 CrossRef CAS PubMed; (c) W. Liu, H. Cao, H. Zhang, H. Zhang, K.-H. Chung, C. He, H. Wang, F.-Y. Kwong and A.-W. Lei, J. Am. Chem. Soc., 2010, 132, 16737 CrossRef CAS PubMed; (d) J. Zhao, Y. Zhao and H. Fu, Angew. Chem., Int. Ed., 2011, 50, 3769 CrossRef CAS PubMed.
- For recent reviews, see: (a) I. Thomé, A. Nijs and C. Bolm, Chem. Soc. Rev., 2012, 41, 979 RSC; (b) R. H. Crabtree, Chem. Rev., 2012, 112, 1536 CrossRef CAS PubMed; (c) A. Bhunia, S. R. Yetra and A. T. Biju, Chem. Soc. Rev., 2012, 41, 3140 RSC; (d) For selected examples, see: N. Leadbeater and M. Marco, Angew. Chem., Int. Ed., 2003, 42, 1407 CrossRef CAS PubMed; (e) R. Arvela, N. Leadbeater, M. Sangi, V. Williams, P. Granados and R. Singer, J. Org. Chem., 2005, 70, 161 CrossRef CAS PubMed; (f) R. K. Arvela and N. E. Leadbeater, J. Org. Chem., 2005, 70, 1786 CrossRef CAS PubMed; (g) S. Buchwald and C. Bolm, Angew. Chem., Int. Ed., 2009, 48, 5586 CrossRef CAS PubMed; (h) N. Leadbeater, Nat. Chem., 2010, 2, 1007 CrossRef CAS PubMed; (i) Z. Gonda, G. L. Tolnai and Z. Novák, Chem. – Eur. J., 2010, 16, 11822 CrossRef CAS PubMed; (j) J. Bonnamour, M. Piedrafita and C. Bolm, Adv. Synth. Catal., 2010, 352, 1577 CrossRef CAS; (k) A. Studer and D. P. Curran, Angew. Chem., Int. Ed., 2011, 50, 5018 CrossRef CAS PubMed; (l) Y. Ji, P. Li, X. Zhang and L. Wang, Org. Biomol. Chem., 2013, 11, 4095 RSC.
- (a) E. von Angerer, N. Knebel, M. Kager and B. Ganss, J. Med. Chem., 1990, 33, 2635 CrossRef CAS; (b) C. Biberger and E. von Angerer, J. Steroid Biochem. Mol. Biol., 1998, 64, 277 CrossRef CAS; (c) M. Medarde, A. C. Ramos, E. Caballero, P.-L. R. De Clairac, J. L. Lopez, D. G. Gravalos and A. S. Feliciano, Bioorg. Med. Chem. Lett., 1999, 9, 2303 CrossRef CAS; (d) G. I. Stevenson, A. L. Smith, S. Lewis, S. G. Michie, J. G. Neduvelil, S. Patel, R. Marwood, S. Patel and J. L. Castro, Bioorg. Med. Chem. Lett., 2000, 10, 2697 CrossRef CAS.
- (a) L. Chu, M. H. Fisher, M. T. Goulet and M. J. Wyvratt, Tetrahedron Lett., 1997, 38, 3871 CrossRef CAS; (b) C. A. Merlic and D. M. McInnes, Tetrahedron Lett., 1997, 38, 7661 CrossRef CAS; (c) A. L. Smith, G. I. Stevenson, S. Lewis, S. Patel and J. L. Castro, Bioorg. Med. Chem. Lett., 2000, 10, 2693 CrossRef CAS.
- (a) R. L. Hudkins, J. L. Diebold and F. D. Marsh, J. Org. Chem., 1995, 60, 6218 CrossRef CAS; (b) M. Amat, S. Hadida, G. Pshenichnyi and J. Bosch, J. Org. Chem., 1997, 62, 3158 CrossRef CAS PubMed; (c) C. N. Johnson, G. Stemp, N. Anand, S. C. Stephen and T. Gallagher, Synlett, 1998, 1025 CrossRef CAS PubMed.
- H.-C. Zhang, H. Ye, K. B. White and B. E. Maryanoff, Tetrahedron Lett., 2001, 42, 4751 CrossRef CAS.
- The reaction solution was analysis by ICP-MS, and the determination data indicated that Cu and Fe are less than 0.27 ppm, and Pd, Sn, Ni, Co, Ru and Rh are less than 0.03 ppm.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra00603h |
| This journal is © The Royal Society of Chemistry 2014 |