Liquid crystal (LC) monolayer on Indium Tin Oxide (ITO): structural and electrochemical characterization†
S. Umadevi*,
V. Ganesh and
Sheela Berchmans
Electrodics and Electrocatalysis (EEC) Division, CSIR-Central Electrochemical Research Institute (CECRI), Karaikudi 630 006, Tamilnadu, India. E-mail: umadevilc@gmail.com; Fax: +91 4565 227779; Tel: +91 4565 227551
First published on 25th March 2014
Abstract
A simple but effective approach to modify transparent Indium Tin Oxide (ITO) substrate with a monolayer of thermotropic liquid crystal (LC) compound containing a terminal carboxylic group and their structural and electrochemical characterization are described. LC compounds employed for monolayer preparation exhibit smectic and nematic phases in bulk. A two-step process was adopted for the immobilization of the LC compound on an ITO electrode which involves the initial formation of a mercaptopropyltrimethoxy silane (MPS) self-assembled monolayer (SAM), followed by the condensation of LC acid onto it through a N,N′-dicyclohexylcarbodiimide (DCC) mediated coupling reaction. Step-wise characterization of the wetting behavior and surface morphology of the SAMs was carried out through contact angle measurements (CA) and atomic force microscopy (AFM) studies, respectively. The quality of the SAM was further assessed by studying the electron transfer process across the modified electrode–electrolyte interface by employing electro-chemical techniques namely cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) using potassium ferro/ferricyanide as a redox probe. As indicated from CV and EIS studies, both MPS modified and LC functionalized ITO substrates exhibit excellent blocking behaviour towards the electron transfer reaction across the electrode–electrolyte interface confirming the presence of dense, well-packed monolayer films. Very high charge transfer resistance (Rct) values and lower rate constant values by up to 4 orders of magnitude compared to bare ITO as determined for both the SAMs from impedance spectroscopy measurements, additionally support the formation of high quality monolayer films. The method described is highly reproducible and the resulting SAMs are stable for several months. Further, versatility of these LC SAM modified ITO electrodes for orientation of the bulk LC sample is also analysed and preliminary results are presented.
Introduction
Over the past decade, Indium Tin Oxide (ITO) has increasingly gained significant importance as a promising substrate material for various applications.1–8 This is due to many interesting properties such as good conductivity, enhanced optical transparency, stability under physiological conditions and the option of tuning the surface properties through chemical modification intrinsically associated with ITO. Self assembled monolayers (SAMs) of many interesting molecules have been formed on ITO as a way to tailor the surface properties and to create functional surfaces.9–13 Among different molecules employed for SAM formation, organic silanes have attracted much attention due to ease of preparation, high thermal and mechanical stability of the resulting SAM and more importantly, their compatibility with Si based technology.1–5,8 Several characterization methods such as microscopic (AFM, scanning tunneling microscopy), spectroscopic (X-ray photoelectron spectroscopy) and electrochemical techniques have been employed to study the structural and morphological features of these silane SAM modified ITO electrodes. Among these, electrochemical techniques namely cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) play a key role to analyze the quality of SAM in terms of electron transfer (ET), surface coverage and defect area.14,15 Most of such studies reported so far were carried out on alkylsilane-based SAMs on ITO.1,2,8LCs, on the other hand, are self organized supramolecular structures which possess order as well as mobility at the molecular and nanoscale level.16,17 Thermotropic LCs are compounds which exhibit liquid crystalline phase on heating a solid or by cooling an isotropic liquid. LCs show interesting optical and electronic properties that can be easily manipulated by external stimuli, the behaviour which made them to find wide applications in display technology.18 In addition, they are also used in sensing, biomedical applications, photonics etc.19–21 Owing to their intrinsic molecular structure which consists of rigid aromatic core and flexible aliphatic chains, LCs have greater potential to form high quality SAMs. Indeed, such superior SAMs have been formed and studied on gold substrates.22 On contrary to gold electrode (which is opaque), chemically modifying ITO substrates with appropriate LC molecules would provide a potential platform to study the unique properties of bulk LCs on a transparent, conductive surface; which permits multiple parameter measurements by employing optical and electrical techniques. In addition to being serving as a base for the alignment of related bulk LCs, these SAMs also have a greater potential as dielectrics for organic thin film devices which is an intensively investigated area. Understanding the fundamental properties of these LC covered ITO electrodes such as structure, electron transfer processes at the interface, charge transfer resistance, surface coverage etc. are highly significant for the effective use of these electrodes in possible applications. However, it is interesting to point out here that, so far, to the best of our knowledge, there are no reports concerning the SAMs of molecules, which show liquid crystalline phase in bulk.
Herein, we report the first examples of LC monolayer modified ITO electrodes and their structural and electrochemical characterization. A simple rod-like thermotropic mesogen (1, shown below) with a carboxylic group on one terminal and a methyl terminated alkoxy chain on another end, exhibiting smectic C and nematic phases, was employed for SAM preparation. A two-step process was adopted to prepare reproducible, stable, highly compact monolayers of these acid terminated mesogens on oxide substrates. In the first step, a SAM of a bifunctional molecule mercaptopropyltrimethoxy silane (MPS) was formed on the pre-treated ITO substrate. In the second step, LC compound was immobilized onto the thiol terminated SAM through a simple DCC coupling reaction. Step-wise structural and electrochemical characterization of the resulting modified ITO electrodes was carried out by employing CA measurement, AFM studies and CV, EIS techniques, respectively. Two mesogens with varying chain length (n = 8 and n = 14) were synthesized and employed for SAM formation to analyze the influence of chain length on electrochemical properties. Preliminary investigation on usefulness of these SAM modified ITO electrodes for bulk LC sample alignment was also carried out.
Experimental
Materials
All the chemicals were obtained from commercial sources and used without further purification. A single-side coated (200–500 nm thick) ITO electrodes with a sheet resistance of Rs = 4–8 Ω were purchased from Delta Technologies Limited, Stillwater, MN, USA. High purity deionised water (Millipore, 18 MΩ) was used to prepare all the aqueous solutions and to rinse the glass wares prior to the experiments.Characterization of LC compounds
Chemical structure of the LC compounds was confirmed through a combination of infrared (IR) and nuclear magnetic resonance (NMR) spectroscopy. IR spectra were recorded on a Bruker Tenser 27 FTIR spectrometer (Bruker Optic GmbH, Germany) using KBr pellets. 1H NMR spectra were recorded on a Bruker Avance 400 MHz spectrometer (Bruker, Switzerland) using tetramethylsilane as an internal standard. The LC properties of the synthesized compounds were investigated using polarizing optical microscopy (POM), differential scanning calorimetry (DSC) and X-ray diffraction studies (XRD). The textural observations were carried out using a Olympus BX50 POM (Olympus Co., Japan) equipped with a Mettler FP92 heating stage and a Mettler FP82HT central processor. The transition temperatures and associated enthalpy values were obtained from thermograms recorded on a Perkin-Elmer (Model Pyris 1) DSC, which was calibrated using pure indium (156.6 °C; ΔH = 28.56 J g−1) as a standard. XRD studies on powder samples were carried out using PANalytical, Empyrean diffractometer using Cu-Kα (λ = 1.54 Å) radiation. The samples were held in Lindemann capillaries with a diameter of 1 mm (Hampton Research, Aliso Viejo, CA, USA). The diffraction patterns of the samples were collected on a two-dimensional image plate (Marresearch, GmbH, Germany).SAM formation and LC immobilization on ITO electrodes
Before SAM formation, ITO electrodes were cleaned thoroughly to activate the surface hydroxyl groups. This pre-treatment process involved rinsing the ITO strips in water and acetone ultrasonically for 15 minutes each. Thereafter, the electrodes were immersed in a solution containing a mixture of hydrogen peroxide, liquid ammonia and water in the ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 respectively, at 70 °C for 30 min. Then the electrodes were washed with a copious amount of Millipore water, dried in a stream of nitrogen and immediately used for surface functionalization with MPS and the subsequent characterization. For SAM formation, a 1 mM solution of the MPS was prepared in toluene and the pre-treated, dried ITO slides were immersed in this solution for an overnight. After this period, the SAM modified electrodes were washed several times with toluene and then rinsed with excess Millipore water. After drying under nitrogen, these SAM modified slides were straight away used for surface characterization. Each time a minimum of two slides were employed for MPS immobilization. One of them was used to confirm the SAM formation through contact angle and CV techniques. Following the confirmation of good quality SAM, the second slide was removed from toluene solution and used for condensation with LC acid after washing with excess toluene and drying under nitrogen. The condensation was carried out by immersing the slide in a septum covered round bottom flask containing a mixture of a suitable LC acid, DCC and 4-(N,N-dimethylamino)pyridine as a catalyst in dry dichloromethane. A proper care was taken to expose the monolayer covered area of the electrode into the solution and a mild stirring was allowed for a homogenous mixing of the reagents without disturbing the electrode surface area. After about 12–14 hours, the electrode was removed from the solution, rinsed with excess dichloromethane, dried under nitrogen and straight away used for structural and electrochemical characterization.
5 respectively, at 70 °C for 30 min. Then the electrodes were washed with a copious amount of Millipore water, dried in a stream of nitrogen and immediately used for surface functionalization with MPS and the subsequent characterization. For SAM formation, a 1 mM solution of the MPS was prepared in toluene and the pre-treated, dried ITO slides were immersed in this solution for an overnight. After this period, the SAM modified electrodes were washed several times with toluene and then rinsed with excess Millipore water. After drying under nitrogen, these SAM modified slides were straight away used for surface characterization. Each time a minimum of two slides were employed for MPS immobilization. One of them was used to confirm the SAM formation through contact angle and CV techniques. Following the confirmation of good quality SAM, the second slide was removed from toluene solution and used for condensation with LC acid after washing with excess toluene and drying under nitrogen. The condensation was carried out by immersing the slide in a septum covered round bottom flask containing a mixture of a suitable LC acid, DCC and 4-(N,N-dimethylamino)pyridine as a catalyst in dry dichloromethane. A proper care was taken to expose the monolayer covered area of the electrode into the solution and a mild stirring was allowed for a homogenous mixing of the reagents without disturbing the electrode surface area. After about 12–14 hours, the electrode was removed from the solution, rinsed with excess dichloromethane, dried under nitrogen and straight away used for structural and electrochemical characterization.
Structural and electrochemical characterization of SAM modified ITO electrodes
Wettability of the monolayer films was studied through contact angle (CA) measurement using water static sessile drop method. The measurements were carried out at room temperature by employing contact angle equipment obtained from AST products Inc., USA, having an automated model of VCA Optima XE. Water CAs on the modified ITO substrates were measured by dispensing a 3 μL drop of water on the sample surface and imaging the droplet immediately, within 10 seconds of contact with the surface. From these recorded images, CAs were determined through the automated software of VCA Optima XE. The CA measurements were performed at four different locations on each sample and the values reported are the average of those four measurements.In addition, surface morphology of both bare ITO and functionalized ITO electrodes was investigated using AFM imaging and these experiments were performed by employing PicoSPM – Picoscan 2100 (Molecular Imaging, USA) instrument using NSC16 ultrasharp silicon cantilever tip (tapping mode). The pre-treated bare ITO and modified ITO electrodes were dried under a stream of nitrogen and imaged under ambient conditions.
Barrier property of the SAMs towards electron transfer reaction across the modified electrode–electrolyte interface was evaluated by employing CV and EIS techniques using potassium ferro/ferricyanide as a redox probe. Experimental details used for CV and EIS studies are reported earlier and a similar procedure was followed.8 Electrochemical impedance analyzer, Model 6310, EC&G instruments obtained from Princeton Applied Research, USA and an AUTOLAB instrument received from The Netherlands were employed for CV and EIS studies respectively. CV experiments and their analysis of the data were carried out using echem software provided by EG&G. The potential ranges and scan rates used for the study are shown in respective diagrams. The equivalent circuit fitting analysis of the impedance data was carried out using Zsimpwin Software (EG&G) developed on the basis of Boukamp's model. From the impedance data useful parameters such as charge transfer resistance (Rct) values of the SAM modified electrodes, surface coverage (θ) of the monolayers and other kinetic parameters were determined.
Results and discussion
A simple, reproducible strategy to anchor methyl terminated LC acids onto transparent ITO substrates resulting in a highly organized hydrophobic surface is described. A graphical representation of the process of functionalizing the ITO substrates with LC acids is shown in Scheme 1. In the first step, a SAM of MPS was formed on the pre-treated ITO substrate. Formation of monolayer of organic silanes on pre-treated ITO surface is a well-known phenomenon wherein, the silane group of the organic compound condenses onto the free hydroxyl groups on the pre-treated surface of the electrode resulting in strong siloxane bonds. Generally, trichlorosilane or trimethoxysilane compounds are employed for the SAM formation on oxide surfaces among which trimethoxysilane derivatives are found to produce dense and compact monolayers due to their higher grafting density.2 Further, there is a possibility of additional condensation between the methoxysilane groups of the adjacent molecules in these monolayers resulting in a highly stable three dimensional siloxane network which provides strong thermal and mechanical stability to the resulting SAM. It is worth pointing out here that the pre-treatment process also has a significant influence on the density and homogeneity of the SAM formation. After confirming the formation of a good quality MPS monolayer on ITO substrate, mesogen (1) was immobilized onto thiol terminated MPS monolayer using a simple DCC coupling reaction (through a thioester linkage). | ||
| Scheme 1 A schematic representation of the method of immobilizing LC acids onto the ITO surfaces. (a) Pre-treated ITO (b) MPS-SAM modified ITO and (c) LC functionalized ITO substrate. | ||
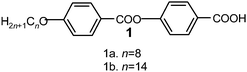
Rod-shaped thermotropic LC compound 1 employed for SAM formation was prepared following a synthetic pathway shown in Scheme 2. According to the Scheme, an appropriate alkoxy benzoic acid was condensed with benzyl 4-hydroxybenzoate to yield a two-ring ester. In the next step, benzyl group was cleaved using catalytic hydrogenolysis, resulting in the required acid. Two compounds with different chain lengths (1a, n = 8 and 1b, n = 14) were prepared and the mesomorphic properties of these compounds were investigated using DSC and POM studies. Both the compounds are enantiotropic mesomorphic and exhibit a smectic C (SmC) and a nematic (N) phase which is confirmed through textural observations and XRD studies. The phase transition temperatures obtained for compounds 1a and 1b, both on heating and cooling at a rate of 10 °C min−1 are summarized in Table 1.
| S. no. | Compound | n | Transition temperatures |
|---|---|---|---|
| a Cr – crystalline phase, SmC – smectic C phase, N – nematic phase, I – isotropic phase. | |||
| 1 | 1a, heating | 8 | Cr 141.0 SmC 177.5 N 236.5 I |
| 2 | 1a, cooling | 8 | I 232.0 N 168.0 SmC 129.0 Cr |
| 3 | 1b, heating | 14 | Cr 120.5 SmC 214.5 N 218.0 I |
| 4 | 1b, cooling | 14 | I 211.0 N 206.8 SmC 100 Cr |
As seen from the table, compound 1a exhibits a wider N phase range (59 °C) as compared to SmC phase (36.5 °C), while compound 1b with a longer terminal chain displays a wider thermal range for the SmC phase (94 °C) and a very short thermal range for the N phase (3.5 °C). This observation is in accordance with the fact that Sm phases are stabilized over N phase for longer alkyl chain derivatives due to the segregation of aromatic and aliphatic parts. In addition, a large hysteresis in transition temperatures can be noted from the table. Since extra care was taken to ensure the purity of the samples, the observed hysteresis can be attributed to the probable degradation of the LC samples with carboxylic acid terminal group at higher temperatures. DSC thermograms recorded for compounds 1a and 1b are provided in electronic supplementary information (Fig. S1 and S2, ESI†). On cooling from isotropic phase and observing under POM, compound 1 sandwiched between normal glass slides exhibited a marble texture in the higher temperature phase which transformed to a grainy texture upon phase transition. In addition to the marble texture, a schlieren texture with two and four brush defects was also observed in a different region of the sample. Furthermore, compound 1 placed between two glass slides treated for homeotropic alignment exhibited no birefringence in the higher temperature phase and showed a schlieren texture with only four brush defects in the lower temperature phase. These observations suggest that higher temperature phase is N and lower temperature phase is SmC phase. The photomicrographs recorded for compound 1a showing the texture of nematic and SmC phases in untreated glass slides, obtained on cooling from the isotropic liquid are shown in Fig. 1. The LC phases were further characterized through XRD studies. A diffuse pattern was obtained in the higher temperature phase and periodic reflections in the ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/2 were recorded in the lower temperature phase (in small angle regions) of compound 1 confirming the presence of N and SmC phase respectively. Both the mesophases exhibited a diffuse reflection in the wide angle region suggesting the liquid-like in-plane order. X-ray diffractograms obtained for compounds 1a in SmC and N phase are provided in the ESI (Fig. S3 and S4† respectively).
1/2 were recorded in the lower temperature phase (in small angle regions) of compound 1 confirming the presence of N and SmC phase respectively. Both the mesophases exhibited a diffuse reflection in the wide angle region suggesting the liquid-like in-plane order. X-ray diffractograms obtained for compounds 1a in SmC and N phase are provided in the ESI (Fig. S3 and S4† respectively).
These LC compounds were employed for the immobilization upon MPS monolayer modified ITO substrate through a thioester linkage.
Contact angle (CA) measurements
CA measurements were carried out to study the surface wettability of the SAM functionalized substrates using sessile drop method. A 3 μL water droplet was placed on the sample surface and an image of the droplet was taken within 10 s of placement of the droplet. An equilibrium water static CA of 62 ± 1° was measured for the as-received ITO surfaces which decreases considerably to 20 ± 2° upon surface pre-treatment. Such a lower CA value obtained for the pre-treated ITO surface indicates the more hydrophilic nature of the surface owing to the induction of a large number of hydroxyl groups on the electrode surface available for condensation. After formation of MPS monolayer, the CA value was found to increase significantly. An equilibrium CA of 73 ± 1° was recorded for MPS modified ITO surface. Comparable values were reported in literature (76 ± 1°) for an analogous SAM on ITO electrode.8 The water static CA increased further upon LC immobilization, indicating the formation of a more hydrophobic surface. Average CA values of 94 ± 1° and 88.5 ± 1° were measured for SAMs of compounds 1a and 1b, respectively. The images of water static CAs measured for the SAMs of MPS and LC compounds 1a and 1b are shown in Fig. 2. The CA value obtained for the monolayer films of MPS (73 ± 1°) indicate a slightly hydrophilic surface due to the presence of a polar thiol terminal group. On the other hand, LC compound with a methyl end-terminal group displayed a relatively higher CA values (94 ± 1°, 88.5 ± 1°) suggesting the successful condensation of LC on to MPS monolayer resulting in an ordered hydrophobic SAM. Similar higher values for static water contact angle are reported in literature for methyl terminated hydrophobic SAMs.8 It is interesting to point out here that compound 1b with a relatively longer terminal alkyl chain exhibits a slightly lower CA value compared to the monolayer film of compound 1a on ITO electrode. These results of CA studies support the strong influence of molecular structure on the SAM formation on ITO substrates using silanes and their surface wettability. Alternatively, the changes in the CA values can also provide valuable information regarding the structural changes on the functional surfaces. | ||
| Fig. 2 Static water contact angle images recorded for (a) MPS modified ITO, (b) 1a immobilized ITO and (c) 1b immobilized ITO surfaces. | ||
Atomic force microscopy (AFM) studies
Surface topography of bare and modified ITO electrodes was studied using an atomic force microscope. Fig. 3 shows the representative AFM images of the surfaces of bare ITO, MPS modified ITO and 1a-LC immobilized ITO electrodes and their corresponding 3D images. | ||
| Fig. 3 AFM images (1 μm × 1 μm) and their corresponding 3D pictures recorded for (a) bare ITO, (b) MPS modified ITO and (c) 1a-LC modified ITO substrates. | ||
As seen from Fig. 3a, bare ITO displayed a homogeneous domain structure on the electrode surface with a root-mean-square (rms, Rq) roughness value of 0.86 nm. Upon MPS immobilization, morphology of the ITO changed considerably from domain-like structure to a granular structure as shown in Fig. 3b (Rq = 2.09 nm). There was further change in the surface structure of ITO upon LC immobilization. Fig. 3c presents the larger granular surface topography of LC immobilized ITO substrate with a rms roughness value of 0.75 nm. It can be clearly seen from the images that the morphology of the LC–ITO is significantly different from that of the MPS–ITO thus implying the successful condensation of LC molecules on MPS-SAM modified ITO surface. Further, the image also illustrates that the LC monolayer film on ITO is complete, dense and homogenous.
Electrochemical characterization
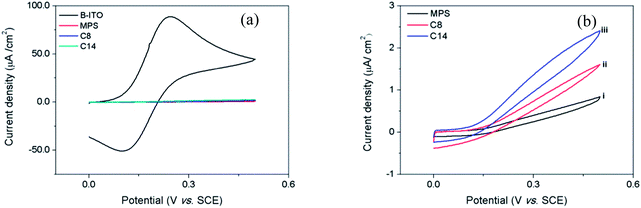
Overall, CV studies clearly indicate the formation of uniform, well-ordered compact monolayer films of MPS as well as LC compounds 1a and 1b on ITO electrode.
The MPS monolayer displayed a larger semicircle compared to that of the LC monolayer films confirming its superior barrier property over LC SAMs. In addition, SAM of LC 1b showed a slightly smaller semicircle in comparison to the SAM of compound 1a indicating its reduced blocking behaviour. These observations clearly support the results obtained in CV experiments (described earlier). Nevertheless, the outcome of EIS studies provided a further proof for the formation of MPS monolayer on ITO and the successful chemical anchoring of LCs onto it.
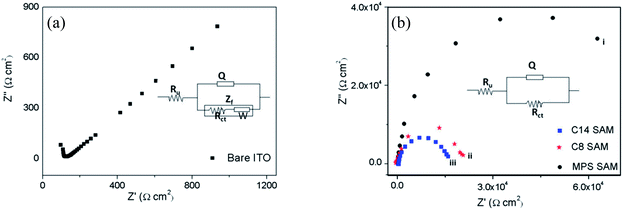
Analysis of impedance data
Analysis of the impedance data obtained for the SAM functionalized electrodes would provide important information regarding the monolayer formation and the distribution of pinholes and defects within the monolayer. The impedance values are fitted to a standard Randle's equivalent circuit (insets in Fig. 5a and b) comprising of a parallel combination of constant phase element (CPE) represented by Q and a faradaic impedance, Zf in series with the uncompensated solution resistance, Ru. The faradaic impedance, Zf is a series combination of charge transfer resistance, Rct and the Warburg impedance, W in the case of bare ITO electrode. For the SAM modified electrodes, the Zf consist of only charge transfer resistance, Rct. The Rct values for bare ITO electrode and SAM modified electrodes are determined by equivalent circuit fitting method using the impedance data. These values are shown in Table 2. Using Rct values, surface coverage of the monolayer films on ITO electrodes is calculated by employing eqn (1),| θimp = 1 − (Rct/R′ct) | (1) |
| Sample | Rct (Ω cm2) | Surface coverage (θimp) | Real/apparent rate constant kapp (cm s−1) |
|---|---|---|---|
| Bare ITO | 17.6301 | — | 0.015104 |
| MPS | 8.3166 × 104 | 0.9997 | 0.3201 × 10−5 |
| 1a | 0.7139 × 104 | 0.9975 | 3.7300 × 10−5 |
| 1b | 0.4128 × 104 | 0.9957 | 6.4507 × 10−5 |
Additionally, heterogeneous rate constant values for the ET process of [Fe(CN)6]3−/4− redox couple are determined for bare ITO and SAM modified ITO electrodes using eqn (2),
| kapp = RT/n2F2RctAC | (2) |
It can be noted from the table that the SAM modified electrodes exhibit higher Rct values (4 orders of magnitude) compared to that of bare ITO electrode due to the inhibition of electron transfer by the formation of monolayer films. While both the MPS and LC monolayer films displayed same order of magnitude for the Rct values, the LC films showed a slightly reduced values compared to that of MPS-SAM. Among LC SAMs, monolayer of 1b has a lower value compared to that of 1a, the difference being very small (0.3011). These minor changes in the Rct values of the functionalized ITO substrates point to a small variation in their blocking behaviour which is in the order MPS > 1a > 1b. Also, it can be seen from the table that the surface coverage (θ) values calculated from the impedance data are >0.99 for all the monolayers indicating the formation of dense, highly-ordered, compact monolayers on ITO electrodes. Additionally, as noted from the table, the rate constant values obtained for SAM modified ITO electrodes are much lower compared to that of bare ITO electrode. These lower values are due to the resistance offered by the monolayer for the ET process which slows down the kinetics. Remarkably, kapp values obtained for both MPS and LC coated ITO electrodes are almost 4 orders of magnitude lower when compared to that of bare ITO electrode signifying their potential blocking ability towards the electron transfer across the modified electrode–electrolyte interface. It is worth pointing out here that very recently electrochemical characterization of the MPS monolayer on ITO electrode has been reported.8 The Rct, θimp and kapp values reported by the authors for the MPS-SAM are very similar to the values obtained by us for the same SAM on ITO electrode.
LC alignment studies
These LC SAM modified ITO electrodes were further explored for their ability to orient bulk LCs. Alignment effects of the LC coated ITO electrodes were studied under POM by employing commercially available sample E7 (from MERCK). E7 exhibits a room temperature nematic phase and turns to isotropic on heating to 60 °C (Tg −62 °C N 60 °C I). Home-made LC cells were prepared by using two modified ITO plates separated by a spacer. E7 was filled into the cell through capillary action by heating the cell to its isotropic temperature. Thereafter, LC cell was slowly cooled to room temperature and the textural observation was carried out under POM. Fig. 6 shows the images of the nematic phase in LC cells made up of (a) untreated ITO plates (8.5 μm) and (b) LC coated ITO plates (8.35 μm). Birefringent texture of the nematic phase under crossed polarisers in Fig. 6a indicates a random orientation of the director of LC molecules in untreated ITO slides and complete extinction in Fig. 6b suggests an uniform homeotropic (normal to the surface) alignment of LC director in LC SAM modified ITO plates. These observations clearly demonstrate the aligning effect of LC SAM modified electrodes in orienting the director of analyte LC. Further, the uniform orientation of bulk LC sandwiched between SAM modified surfaces provide evidence for the homogeneous molecular ordering of SAM on ITO surfaces.From these studies, it is confirmed that these SAMs represent a superior quality monolayers on oxide surfaces and the simple strategy described here for the SAM formation is an efficient method to functionalize ITO substrates with LC molecules containing acid terminal groups. Apart from serving as supporters to obtain a uniform, well-oriented LC films, these chemically modified ITO electrodes also have a greater potential as dielectrics for applications in organic thin film devices. In addition to their orienting effect, detailed studies on influence of these SAM modified electrodes on other LC properties such as dielectric behaviour, switching properties etc. are currently under progress.
Conclusions
A simple approach to functionalize the transparent ITO electrodes with LC molecules resulting in a high quality SAM with a surface coverage of >0.999 is demonstrated. Initial formation of a uniform, compact monolayer film of thiol terminated MPS molecules on ITO serves as a soft template for the homogeneous immobilization of methyl terminated LC acids through DCC coupling reaction yielding a highly organized hydrophobic surface. As observed from CV and EIS, the LC functionalized ITO substrates exhibit significant blocking behaviour towards the electron transfer reaction across the electrode–electrolyte interface confirming the presence of dense, well-packed LC monolayer films. The heterogeneous rate constant values calculated for SAM modified electrodes are almost 4 orders of magnitude lower when compared to the bare ITO electrode. Additionally, the LC coated ITO substrates are found to be very effective in orienting the bulk LC sample.Acknowledgements
Author SU would like to thank Department of Science and Technology (DST), India for financial support under Women Scientist Scheme (WOS-A) having project number CS/SR-124/2011. Authors gratefully acknowledge Central Instrumentation Facility (CIF) of CSIR-CECRI for access and assistance with AFM measurements. Authors also thank Mrs K. N. Vasudha, RRI, Bangalore for help in DSC and XRD studies. Special thanks to Prof. B. K. Sadashiva, RRI, Bangalore for useful discussions.Notes and references
- I. Markovich and D. Mandler, J. Electroanal. Chem., 2000, 484, 194–202 CrossRef CAS; I. Markovich and D. Mandler, J. Electroanal. Chem., 2001, 500, 453 CrossRef.
- H. Hillebrandt and M. Tanaka, J. Phys. Chem. B, 2001, 105, 4270 CrossRef CAS.
- C. K. Luscombe, H. W. Li, W. T. S. Huck and A. B. Holmes, Langmuir, 2003, 19, 5273 CrossRef CAS.
- L. Yang and Y. Li, Biosens. Bioelectron., 2005, 20, 1407 CrossRef CAS PubMed.
- L.-W. Chong, Y.-L. Lee and T.-C. Wen, Thin Solid Films, 2007, 515, 2833 CrossRef CAS PubMed.
- I. Willner and B. Willner, Nano Lett., 2010, 10, 3805 CrossRef CAS PubMed.
- K. Kamada, T. Nakamura and S. Tsukahara, Chem. Mater., 2011, 23, 2968 CrossRef CAS.
- A. Muthurasu and V. Ganesh, J. Colloid Interface Sci., 2012, 374, 241 CrossRef CAS PubMed.
- C. Yan, M. Zharnikov, A. Golzhauser and M. Grunze, Langmuir, 2000, 16, 6208 CrossRef CAS.
- M. S. Kang, H. Ma, H. L. Yip and A. K. Y. Jen, J. Mater. Chem., 2007, 17, 3489 RSC.
- L. W. Chong, Y. L. Lee and T. C. Wen, Thin Solid Films, 2007, 515, 2833 CrossRef CAS PubMed.
- Y. Choi and J. Noh, Mol. Cryst. Liq. Cryst., 2008, 492, 165 CAS.
- D. M. Rampulla, C. M. Wroge, E. L. Hanson and J. G. Kushmerick, J. Phys. Chem. C, 2010, 114, 20852 CAS.
- A. Ulmann, An Introduction to Ultrathin Organic Films: From Langmuir-Blodgett to Self-Assembly, Academic Press, Boston, 1991 CrossRef PubMed; A. Ulmann, Chem. Rev., 1996, 96, 1533 CrossRef PubMed.
- C. J. Miller and I. Rubinstein, Physical Electrochemistry: Principles, Methods and Applications, Marcel Derek, New York, 1995 Search PubMed.
- D. Demus, J. Goodby, G. W. Gray, H.-W. Spiess and V. Vill, Handbook of Liquid Crystals Set, Wiley-VCH, Weinheim, 1998 Search PubMed.
- P.-G. de Gennes, Angew. Chem., Int. Ed. Engl., 1992, 31, 842 CrossRef PubMed.
- M. Bremer, P. Kirsch, M. Klasen-Memmer and K. Tarumi, Angew. Chem., Int. Ed., 2013, 52, 8880 CrossRef CAS PubMed , and references therein.
- J. M. Brake, M. K. Daschner, Y. Y. Luk and N. L. Abbott, Science, 2003, 302, 2094 CrossRef CAS PubMed.
- A. D. Price and D. K. Schwartz, J. Am. Chem. Soc., 2008, 130, 8188 CrossRef CAS PubMed.
- D. C. Zografopoulos, R. Asquini, E. E. Kriezis, A. d'Alessandro and R. Beccherelli, Lab Chip, 2012, 12, 3598 RSC.
- V. Ganesh, S. K. Pal, S. Kumar and V. Lakshminarayanan, J. Colloid Interface Sci., 2006, 265, 195 CrossRef CAS PubMed; V. Ganesh, S. K. Pal, S. Kumar and V. Lakshminarayanan, Electrochim. Acta, 2007, 52, 2987 CrossRef PubMed.
- M. Mansueto, S. Sauer, M. Butschies, M. Kaller, A. Baro, R. Woerner, N. H. Hansen, G. Tovar, J. Pflaum and S. Laschat, Langmuir, 2012, 28, 8399 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Spectral data, DSC plots and X-ray diffractograms obtained for compounds 1a and 1b. See DOI: 10.1039/c4ra00556b |
| This journal is © The Royal Society of Chemistry 2014 |