Continuous synthesis of colloidal chalcopyrite copper indium diselenide nanocrystal inks
Abstract
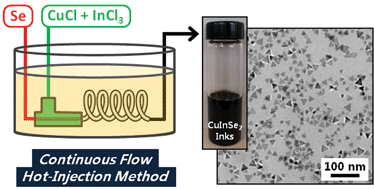
A continuous synthetic method in a micro-tubular reactor is introduced for synthesizing mono-disperse and solution-stable chalcopyrite colloidal copper indium diselenide nanocrystal (CuInSe2 NC) inks with potential scalability. It was found that the morphologies of the CuInSe2 NCs were dependent on the Cu/In/Se composition. The NC morphology changed from spherical to hexagonal to trigonal with increasing In or Se content, whereas trigonal morphologies synthesized at high temperature yielded chalcopyrite CuInSe2 NCs. A laboratory-scale photovoltaic device with 1.9% efficiency under AM1.5G illumination was also fabricated to verify the utility of these inks.

 Please wait while we load your content...
Please wait while we load your content...