Luminol chemiluminescence enhanced by copper nanoclusters and its analytical application
Abstract
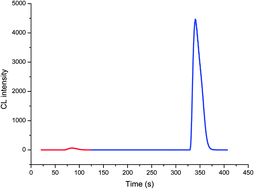
It was found that Cu nanoclusters could enhance the chemiluminescence (CL) emission from the luminol–hydrogen peroxide system in an alkaline medium. Herein, the CL spectra, UV-visible spectroscopy and radical scavengers were investigated to explore the possible enhancement mechanism. The enhanced CL could be attributed to the catalysis of Cu nanoclusters, which effectively catalyzed the decomposition of H2O2 to produce double hydroxyl radicals. The inhibiting effects of some organic compounds were also investigated. Then, the proposed method was successfully applied to determine H2O2 in environmental water samples with satisfactory accuracy and precision.

 Please wait while we load your content...
Please wait while we load your content...