DFT studies for the evaluation of amine functionalized polystyrene adsorbents for selective adsorption of carbon dioxide
Rupa Shantamal Madyal and
Jyotsna Sudhir Arora*
Department of Chemical Engineering, Institute of Chemical Technology, Nathalal Parekh Marg, Matunga, Mumbai 400019, India. E-mail: josharora@gmail.com; Fax: +91-022-33611020; Tel: +91-022-33612013
First published on 23rd April 2014
Abstract
The interaction of carbon dioxide with four amine functionalized polystyrene based adsorbents was investigated by means of density functional theory (DFT). The structures of the adsorbents and CO2–adsorbent complexes were optimized using the B3LYP/6-311++G(d,p) method. The interaction energies, equilibrium distances, charge transfer from the functionalized adsorbent to CO2, highest occupied molecular orbital (HOMO)–lowest unoccupied molecular orbital (LUMO) energy gap and thermochemical parameters of the complexes were evaluated. Weak intermolecular forces were responsible for the interaction between the active centers of functionalized adsorbents and CO2 as confirmed from experimental studies. The interaction energies and vibrational frequencies confirmed stronger interaction of CO2 towards imidazole functionalized polystyrene (PS-imidazole) followed by N-methylpiperazine loaded polystyrene (PS-piperazine) and were found to be the least for the bare adsorbent chloromethylated polystyrene (CMPS). Steric hindrance was found to play a major role in the case of dimethylamine (PS-DMA) and diethanolamine (PS-DEA) functionalized polystyrene during their interaction with CO2. On the basis of the HOMO–LUMO energy gap and calculated density of states, a negligible change in the electronic properties of CO2–adsorbent complexes was observed, indicating a physisorption process. The outcomes of the present theoretical studies are in good agreement with the experimental results and provide detailed insights for understanding the interaction of CO2 with the active center of the functionalized adsorbent.
1 Introduction
The concentration of carbon dioxide (CO2) in the atmosphere has increased by about 30% in the last 50 years and is expected to increase over the next few decades as a result of anthropogenic fossil-fuel combustion.1 Carbon dioxide is considered to be the major contributor to the greenhouse effect and is perceived to be responsible for global warming. Thus, the presence of excess CO2 in earth's atmosphere has resulted in the gradual increase in temperature. Extensive efforts have been focused to develop efficient technologies for CO2 capture, storage (sequestration) and utilization.2,3CO2 capture by liquid amines (primary and secondary) are amongst the most commonly used processes.1,4–6 However, the main drawbacks of the solvent based CO2 absorption are high energy consumption for solvent regeneration accompanied by degradation of the amine leading to generation of waste, thus resulting in the capital loss.1,4,7 Hence, adsorption is considered to be an alternative promising technology for separating CO2 from gaseous mixtures due to its low energy requirement, easy operation and low maintenance.8 It involves binding of a molecule onto the solid surface by strong chemical or weak physical interactions or at times even both and adsorbent regeneration is carried out by either pressure or temperature swing cycles.8–15 However, the development of low cost adsorbent with high CO2 selectivity and adequate adsorption capacity under ambient conditions is an active area of research.16 The solid sorbents such as activated carbon,17 zeolites,18 zeolitic imidazole frameworks (ZIFs),19 single/multi-walled carbon nanotubes,1 nanoporous silica-based molecular basket,20 polymers21–25 and metal–organic frameworks (MOFs)1,7,10 have been used for adsorption of CO2. The recent studies have focused on use of amine functionalized adsorbents for selective separation of CO2 from flue gas mixtures.7,17,20,22–24,26–28 The incorporation of amine functionality on the surface of the adsorbent significantly increases CO2 selectivity and uptake capacity because CO2 is an acidic gas.2
As the quest for the development of CO2-specific adsorbent is continuously increasing, the use of computational studies has become an indispensable tool for understanding the parameters which govern CO2–adsorbent interactions at the molecular level. Panda et al. performed computational studies with a series of ZIFs using different 4,5-functionalized imidazole units, viz., –CH3, –OH, –Cl, –CN, –CHO and –NH2 by applying Universal Force Field (UFF).19 The symmetry and polarizability of the functional group exhibited significant influence on CO2 uptake. MOF,1,7,10 CNT1 and zeolites18 have also been investigated to predict the selective CO2 adsorption using ab initio calculations. Thakur et al. theoretically studied the adsorption of CO2 on graphene surface and reported π interaction of CO2 with the electron rich benzene ring.29 Hussain et al. performed ab initio calculations to study the affinity of CO2 towards 20 naturally occurring amino acids and also proposed amino acids as alternatives to chemical dissolution of CO2.30 Baei et al. theoretically studied the interaction of CO2 with B12N12 nanoclusters using DFT calculations and reported interaction energy of −2.30 kcal mol−1 on physisorption.12 Valenzano et al. reported 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 adsorption complex of CO, N2, and CO2 with the Mg2+ adsorption site of Mg-MOF-74 by weak (dispersion) interactions.11
1 adsorption complex of CO, N2, and CO2 with the Mg2+ adsorption site of Mg-MOF-74 by weak (dispersion) interactions.11
Extensive efforts are being directed from a variety of fields including polymer technology to search better CO2 capturing materials.21–25,31–33 The surface properties of the polymer can easily be tailored with different organic or inorganic ligands. After functionalization of the polymer surface, the accessibility of the amine to capture CO2 is the most important factor to be considered during adsorption. Primary22–24 and secondary amine22,23 functionalized polymeric adsorbents have already been used for CO2 capture which result in the formation of carbamate species. The regeneration of such adsorbents involve energy intensive temperature swing process.22,24,34 However, tertiary amines (in the absence of moisture) do not support formation of carbamate and thus bind CO2 by physical interactions only.28 Recently, Khot et al. carried out CO2 sorption studies with tertiary amine functionalized polystyrene as well as with bare polystyrene.8 The amine functionality is covalently attached to the macroporous surface of polymeric adsorbent after the bead formation. Hence, the surfaces of the pores are expected to be available for adsorption and a molecule like CO2 diffuses through the pores of the adsorbent beads. Further, these amines are neither mobile nor there will be any loss of functionality from the surface during the adsorption and desorption process.
The current work includes theoretical studies of these tertiary amine functionalized adsorbents for interaction with CO2. Since quantum mechanical calculations involving the entire polymeric structure would be difficult and computationally expensive, only the amino functional group covalently attached to the repeating structure of the polymer viz. styrene moiety, was taken for the current study. The calculations were carried out with the objective to gain fundamental insights into equilibrium geometry of the adsorbents and the complexes, interaction energy, charge transfer, band gap, vibrational frequencies and understand the dominant factors responsible for CO2 complexation. This study is expected to be useful for future designing of CO2 specific adsorbents.
2 Computational methodology
The DFT studies were performed using Gaussian 09 package35 for investigating the structural and electronic properties during interaction of CO2 with functionalized adsorbent as well as with the bare polymer, chloromethylated polystyrene (CMPS). The functional considered is Becke's three parameter-Lee–Yang–Parr correlation B3LYP4,5,7 with 6-311++G(d,p)5,36,37 basis set. The geometry optimization of the adsorbents, free CO2 and the CO2–adsorbent complexes was carried out without imposing any initial symmetry restriction. In order to find the most stable equilibrium structure for CO2–adsorbent complex, several initial guess structures were considered based on the position of CO2 close to the electron rich sites of the adsorbent and only the minimum energy structures are further studied. The stationary points were characterized as no imaginary frequencies were obtained during Hessian calculations.Corrections to the interaction energy for all the complexes were evaluated by considering the basis set superposition error (BSSE) with counterpoise correction (CPC) calculation.4,11 Natural population analysis using the Natural Bond Order (NBO) method was performed.4 The charge transfer (QT) from the donor atoms of the amine loaded adsorbents to CO2 during the interaction was evaluated by summing up the electrostatic potential (ESP) charges on CO2 molecule.30 To compare the calculated vibrational frequencies with the experimental values, the calculated frequencies were scaled using a factor of 0.964 ± 0.023.37 The contribution of a group to a molecular orbital was calculated by Mulliken population analysis (MPA). The density of states (DOS) spectra were created by convoluting the molecular orbital information, allowing identification of amine and CO2 centered orbitals with Gaussian curves of full width at half maximum (FWHM) = 0.3 eV. The DOS diagrams for all the adsorbents and the complexes presented in this work were obtained using the Multiwfn program.38
3 Results & discussion
3.1 Molecular electrostatic potential analysis of the adsorbents
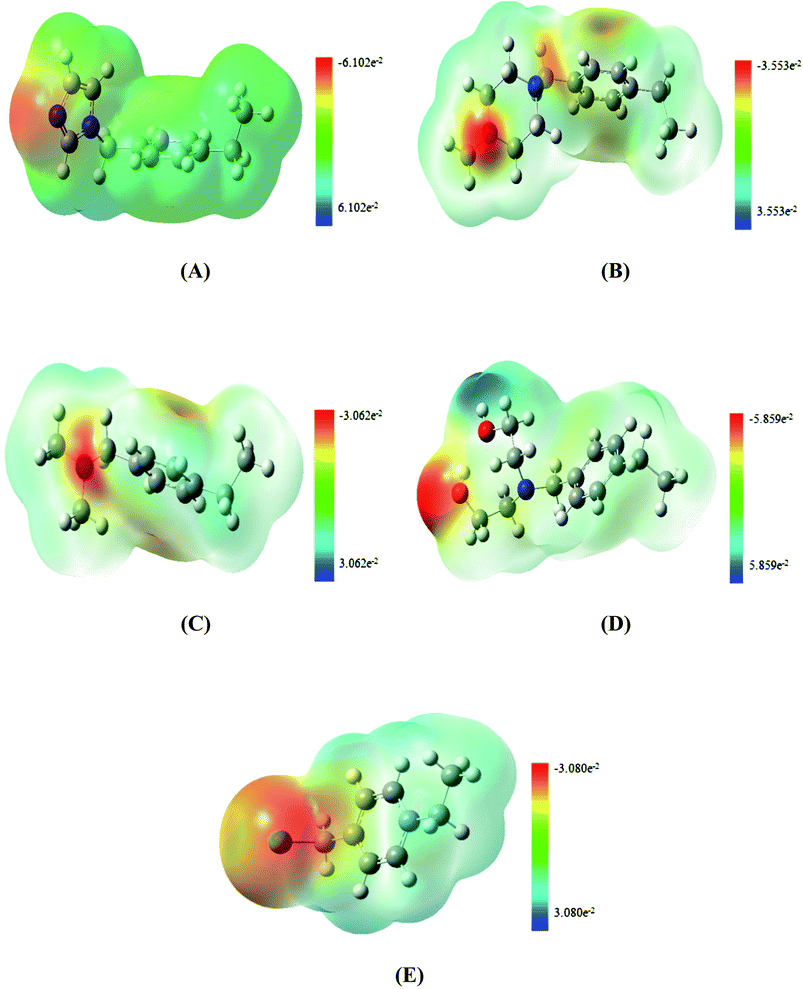
The calculated molecular electrostatic potential surface (MESP) of the optimized structures of the four amine functionalized adsorbents along with CMPS at DFT-B3LYP level are shown in Fig. 1. MESP measures the electrostatic potential onto the isoelectron density surface and simultaneously displays electron and nuclei distribution which provides a good understanding of the relative polarity within the molecule.39 In Fig. 1, the red color refers to the high electron density region (negative ESP) while the blue colored region refers to the positive ESP within the adsorbent. Out of the four amines, imidazole is a heterocyclic aromatic amine consisting of two ‘N’ atoms. Out of two ‘N’ atoms, one has sp2 and other has sp3 character, while the remaining three aliphatic amines (N-methylpiperazine, dimethylamine (DMA) and diethanolamine (DEA)) contain only sp3 nitrogen. For imidazole functionalized polystyrene (PS-imidazole), maximum electron density is localised over the sp2 nitrogen atom (Fig. 1(A)). In N-methylpiperazine (PS-piperazine) and dimethylamine (PS-DMA) functionalized polystyrene, the nitrogen center is electron rich (Fig. 1(B)) and (Fig. 1(C)), respectively. In diethanolamine functionalized polystyrene (PS-DEA), the electron density is found to spread more over the ‘O’ atom of hydroxyl groups rather than ‘N’ of amine (Fig. 1(D)). In the case of CMPS (Fig. 1(E)), the electrons are more localised over the chlorine atom as compared to π-electron rich benzene ring as confirmed from ESP studies. Amongst all the functionalized adsorbents, the electron density lies over either ‘N’ or ‘O’ atoms of the amine and thus, poses suitable sites for preferential adsorption CO2 over other adsorption site viz. the phenyl ring.3.2 Study of CO2–adsorbent complexes: structure and stability
The equilibrium geometries of CO2–adsorbent complexes are given in Fig. 2. The degree of interaction between CO2 and the adsorbents was investigated by calculating the interaction energy ΔEnoCPC as given by eqn (1).| ΔEnoCPC = TEcomplex − (TEadsorbent + TECO2) | (1) |
| ΔECPC,corr = ΔEnoCPC + BSSE | (2) |
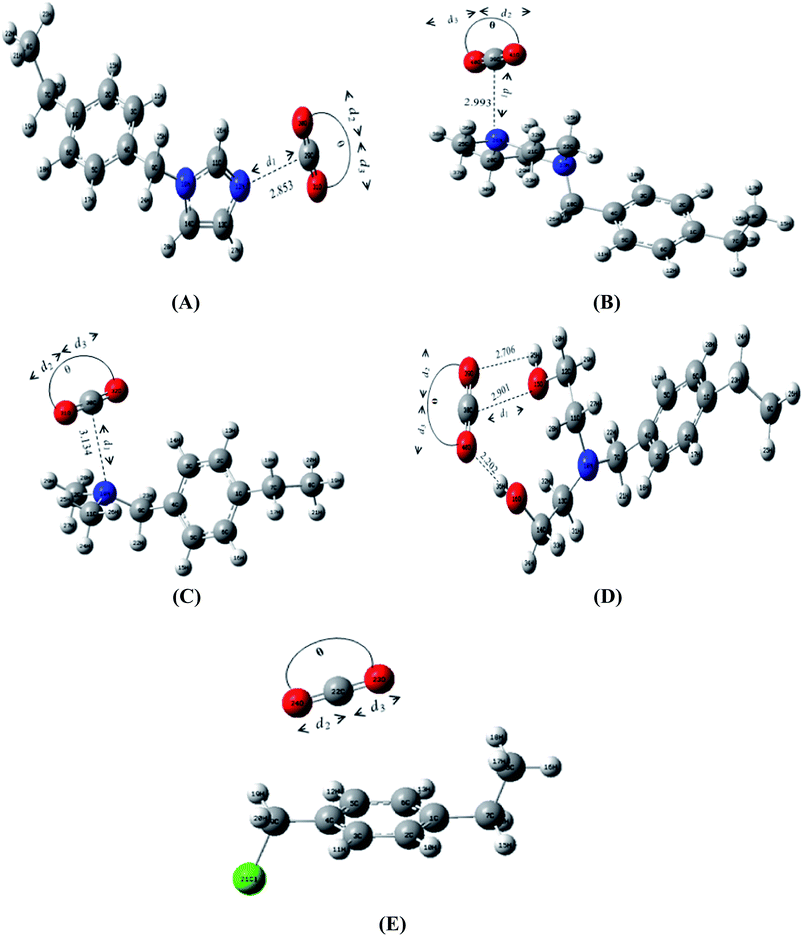 | ||
| Fig. 2 Optimized structures of PS-imidazole + CO2 (A), PS-piperazine + CO2 (B), PS-DMA + CO2 (C), PS-DEA + CO2 (D), and CMPS + CO2 (E) complexes. The calculated values of d1, d2, d3 and θ are given in Table 2. | ||
| Adsorbents | ΔEnoCPC | ΔEBSSE | ΔECPC,corr | Δ(ZPE) | ΔET/R/V | TΔS | ΔG | ΔGa |
|---|---|---|---|---|---|---|---|---|
| a Interaction between Lewis base, pentafluoro phenolate anion and CO2, ΔE = −23.1 kJ mol−1 and the distance between the oxygen of the anion and ‘C’ of CO2, 2.43 Å (Teague et al.).4 | ||||||||
| PS-imidazole | −12.80 | 0.95 | −11.85 | 1.67 | 5.74 | −24.18 | 17.25 | 12.7 |
| PS-piperazine | −8.24 | 1.58 | −6.66 | 2.41 | 5.94 | −28.56 | 30.25 | |
| PS-DMA | −6.57 | 1.49 | −5.08 | 2.78 | 6.02 | −30.38 | 34.10 | |
| PS-DEA | −4.22 | 1.55 | −2.67 | 3.13 | 6.18 | −30.56 | 37.19 | |
| CMPS | −3.56 | 1.51 | −2.05 | 3.24 | 6.29 | −30.77 | 38.26 | |
The smaller values of ΔECPC,corr implies that CO2 experiences weak van der Waals interaction with the adsorbent, thus it is identified as physical in nature.2,40 The thermal and entropic contributions were considered to calculate the free energy of the system which is given by eqn (3),4
| ΔG = ΔECPC,corr + Δ(ZPE) + ΔET/R/V – RT − TΔS | (3) |
The enthalpic contributions to ΔE were indicative of the specific interactions, but the entropic factors seem to negate the advantage of specific interactions. The ΔG values for the functionalized and the bare adsorbent is positive indicating that the interaction between the adsorbent and CO2 is weak as the complexes are destabilized by entropic effects and hence the gas phase reaction is unfavorable at 303 K and 1 bar. A positive change in free energy, however, should not be taken as a sufficient reason for not pursuing a potentially useful reaction involving CO2, the kinetics might indeed be favorable. Hence to achieve significant adsorption, increasing the pressure and/or lowering the temperature may enhance the binding of CO2 with amine functionality. The optimized parameters (ΔE and distances between the interacting centers) from the current study are comparable to the positive free energy results reported by Teague et al.4 (Table 1).
The experimental investigations of CO2 adsorption on these four functionalized adsorbents executed by Khot et al.41 confirmed the physical nature of adsorption. Also, the IR frequencies of the adsorbents after CO2 adsorption showed no changes in the C–N stretching frequency towards 1609 cm−1 and the asymmetric stretching (ν3) of CO2 (2349 cm−1), indicating no carbamate and bicarbonate formation.8 An improved adsorption of CO2 on the amine functionalized adsorbents was observed by Khot et al.8 during the experimental studies performed by increasing the pressure (∼40 bar). Thus, the above methodology correctly predicts the presence of physisorbed CO2 on amine functionalized polystyrene.
The observed trend in interaction energies can be well correlated to the distance between CCO2–donor atom of the functionalized adsorbent (d1). PS-imidazole which exhibits the strongest interaction with CO2 has the lowest CCO2–Namine distance (2.8533 Å), followed by PS-piperazine (2.9933 Å) and PS-DMA (3.1346 Å). This is because the planar structure of imidazole experiences less steric hindrance allowing the sp2 nitrogen atom to move closer to the ‘C’ of CO2 as compared to PS-piperazine and PS-DMA. In the case of PS-DEA, due to the inaccessibility of the ‘N’, electron deficient ‘C’ of CO2 interacts with the electronegative –OH groups of DEA. The ‘O’ atoms of CO2 strongly interact with the electropositive ‘H’ atoms of the alcoholic group thus leading to the formation of a hydrogen bonded structure.42 However with CMPS, CO2 exhibits π-quadrupolar interaction with the π-electron rich phenyl ring. In CMPS–CO2 complex, CO2 is parallel to the ring but slightly displaced from the centre of mass of phenyl ring.25,43
The interaction between CO2 and the donor atom of the amine functionality leads to varying degrees of distortion in carbon dioxide molecule as well as the interacting centers of the adsorbents. The calculated bond length of free CO2 is 1.1608 Å, which is comparable to the experimental bond length (1.161 Å).44 On analyzing the structure of adsorbed CO2, we see lengthening of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond which is closer to the active center (d2 − d0) in all systems when compared to the free CO2 bond length (1.1608 Å) while, the C
O bond which is closer to the active center (d2 − d0) in all systems when compared to the free CO2 bond length (1.1608 Å) while, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond farther away from the active center (d3) is shortened in all the adsorbents (d3 − d0). Summing up the two C
O bond farther away from the active center (d3) is shortened in all the adsorbents (d3 − d0). Summing up the two C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond lengths (d2 and d3), yields the overall length (l) of the CO2 molecule, which can be used in determining elongation of the molecule (l − l0) after interaction, for all the adsorbents. PS-imidazole and PS-piperazine display a higher increase in bond length of physisorbed CO2 as compared to that with PS-DMA and PS-DEA (Table 2) owing to the strong interaction with CO2. Also, the bond lengths of amine functional group changes during the complexation. The Camine–donor atom bond lengths before and after complexation are listed in Table 3.
O bond lengths (d2 and d3), yields the overall length (l) of the CO2 molecule, which can be used in determining elongation of the molecule (l − l0) after interaction, for all the adsorbents. PS-imidazole and PS-piperazine display a higher increase in bond length of physisorbed CO2 as compared to that with PS-DMA and PS-DEA (Table 2) owing to the strong interaction with CO2. Also, the bond lengths of amine functional group changes during the complexation. The Camine–donor atom bond lengths before and after complexation are listed in Table 3.
| Adsorbents | d1 | d2 | d3 | (d2 − d0) × 103 | (d3 − d0) × 103 | (l − l0) × 103 | θ (deg) | QT |
|---|---|---|---|---|---|---|---|---|
a All distances are given in Å. d0 = 1.1608 Å (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond length of unadsorbed CO2). d2 and d3 are C O bond length of unadsorbed CO2). d2 and d3 are C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond length of physisorbed CO2. l0 = 2 × d0 = 2.3216 Å (bond length of CO2 before interaction). l = d2 + d3 (bond lengths of CO2 after interaction). O bond length of physisorbed CO2. l0 = 2 × d0 = 2.3216 Å (bond length of CO2 before interaction). l = d2 + d3 (bond lengths of CO2 after interaction). |
||||||||
| CMPS | 1.1615 | 1.1602 | 0.72 | −0.60 | 0.12 | 179.54 | ||
| PS-imidazole | 2.8533 | 1.162 | 1.1607 | 1.47 | −0.01 | 1.46 | 176.05 | −0.009 |
| PS-piperazine | 2.9933 | 1.1616 | 1.1615 | 0.80 | 0.75 | 1.55 | 176.74 | −0.012 |
| PS-DMA | 3.1346 | 1.1613 | 1.1609 | 0.55 | 0.15 | 0.70 | 177.53 | −0.005 |
| PS-DEA | 2.9013, 2.2023, 2.7061 | 1.1616 | 1.1599 | 0.84 | −0.90 | −0.06 | 178.06 | −0.011 |
| Adsorbents | Bonds | Before complexation | After complexation |
|---|---|---|---|
| PS-imidazole | C(11)–N(12) | 1.3137 | 1.3157 |
| PS-piperazine | C(20)–N(24) | 1.4623 | 1.4662 |
| C(21)–N(24) | 1.4638 | 1.4669 | |
| C(25)–N(24) | 1.4539 | 1.4571 | |
| PS-DMA | C(9)–N(10) | 1.4635 | 1.4706 |
| C(11)–N(10) | 1.4581 | 1.4611 | |
| C(12)–N(10) | 1.4565 | 1.4601 | |
| PS-DEA | O(15)–H(35) | 0.9618 | 0.9626 |
| O(16)–H(36) | 0.9618 | 0.9641 | |
| C(12)–O(15) | 1.4387 | 1.4413 | |
| C(14)–O(16) | 1.4251 | 1.4275 |
Further, CO2 molecule undergoes nonlinear distortion, and the calculated deviation of ∠O–C–O from its linear geometry (180°) in the adsorbed state is relatively small, ∼1–5° (Table 2). The extent of bending of CO2 during complexation is generally considered to be a measure of the basicity of the amine.4 Amongst the amine functionalized adsorbents, PS-imidazole and PS-piperazine reveal maximum change in the ∠O–C–O bond angle as compared to PS-DMA and PS-DEA, which justifies superior contact with the former adsorbents. CMPS which shows the least interaction with CO2 at atmospheric pressure exhibits the lowest bent of ∠O–C–O (179.54°) as compared to the functionalized adsorbents (Table 2). Thus, the extent of distortion of CO2 from the linear geometry and shorter CCO2–donor atom distances (d1) can be correlated to stronger CO2–adsorbent interactions.
3.3 Charge transfer to CO2 from the adsorbent
To understand the electronic effects taking place during the interaction of CO2 with the adsorbents, the charge transfer (QT) to CO2 from amine functionalized adsorbent was investigated and the values are listed in Table 2. For all the CO2–adsorbent complexes, negative QT indicates charge flow from the lone pair of electronegative atoms of the amine functional group to CO2 which is consistent with Lewis type acid–base interaction.30 Maximum charge transfer occurs in PS-piperazine followed by PS-DEA, PS-imidazole and least for PS-DMA. In PS-piperazine, due to the positive inductive effect (+I) of the –CH3 group attached to ‘N’ as well as the chair conformation of the ring makes access for interaction of CO2 with the active center viz., electron rich ‘N’ atom easier and thus maximum electrons are transferred to CO2. In case of PS-DEA, the ‘N’ atom is sterically hindered due to the presence of two ethylene groups which results in the inaccessibility of ‘N’ towards CO2. This causes weak intermolecular hydrogen bonding with ‘O’ of CO2 and thus maximum electronic charge is transferred from ‘O’ of alcoholic group to CO2 resulting in higher QT value.3.4 Vibrational frequency analysis
The change in bond lengths of the interacting species, asymmetric distortion of CO2 as well as change in atomic charges after complexation results in a corresponding change of the dipole moment. These changes significantly affect the vibrational frequencies of the interacting centers.45,46 The vibrational spectral analyses of the interactive centers (‘C’ of CO2 and ‘donor atom’ of amine functionalized adsorbent) before and after complexation as well as the asymmetric stretching vibration of CO2 (v3) are given in Table 4.| Adsorbents | Bonds | Before (cm−1) | After (cm−1) | |||||
|---|---|---|---|---|---|---|---|---|
| C-donor atom | ν3a | ν3b | ν3c | Δν3 | ||||
| Exp. | Cal. | |||||||
a Calculated frequency of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O.b Calculated frequency of C O.b Calculated frequency of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O multiplied with the scaling factor (0.964).c Experimental frequency.8d Simulated frequency of CO2.46 O multiplied with the scaling factor (0.964).c Experimental frequency.8d Simulated frequency of CO2.46 |
||||||||
| CO2 | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O |
2420 | 2333 | 2349d | — | — | ||
| PS-imidazole | C(11)–N(12) | 1535 | 1537 | 2413 | 2326 | 2335 | 14 | 7 |
| PS-piperazine | C(20)–N(24) | 1219 | 1221 | 2411 | 2324 | 2336 | 13 | 9 |
| PS-DMA | C(9)–N(10) | 1115 | 1116 | 2415 | 2328 | 2348 | 1 | 5 |
| PS-DEA | O(15)–H(35), C(12)–O(15) | 3315, 1039 | 3345, 1043 | 2420 | 2333 | 2348 | 1 | 0 |
The simulated frequency values are slightly higher than the experimental values which is due to the fact that the experimental values are anharmonic frequencies while the calculated values are harmonic frequencies.47 Hence, the calculated vibrational frequencies of CO2 (v3) are compared with experimentally determined FTIR frequency values8 of physisorbed CO2 after multiplying with the scaling factor (Table 4). Increase in the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond length of CO2 results in decrease in v3 (red shift).46 As mentioned earlier, PS-imidazole and PS-piperazine show a considerable increase in bond length of CO2 and thus result in the higher v3 shift as compared to PS-DMA and PS-DEA, respectively. A fairly consistent trend is observed between experimental and DFT calculated v3 values.8 The stretching frequency of C(11)
O bond length of CO2 results in decrease in v3 (red shift).46 As mentioned earlier, PS-imidazole and PS-piperazine show a considerable increase in bond length of CO2 and thus result in the higher v3 shift as compared to PS-DMA and PS-DEA, respectively. A fairly consistent trend is observed between experimental and DFT calculated v3 values.8 The stretching frequency of C(11)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N(12) bond in PS-imidazole exhibited a 2 cm−1 shift on complexation with CO2. Similarly, the C(20)–N(24) symmetrical stretching shifted from 1219 to 1221 cm−1 in PS-piperazine, while only 1 cm−1 shift in C(9)–N(10) symmetrical stretching was observed for PS-DMA adsorbent. However, in PS-DEA adsorbent the electron rich oxygen center (O(15)) polarizes more on complexation as indicated by increase in its symmetric stretching frequency (C(12)–O(15) exhibits 4 cm−1 while O(15)–H(35) reveals 30 cm−1 shift).
N(12) bond in PS-imidazole exhibited a 2 cm−1 shift on complexation with CO2. Similarly, the C(20)–N(24) symmetrical stretching shifted from 1219 to 1221 cm−1 in PS-piperazine, while only 1 cm−1 shift in C(9)–N(10) symmetrical stretching was observed for PS-DMA adsorbent. However, in PS-DEA adsorbent the electron rich oxygen center (O(15)) polarizes more on complexation as indicated by increase in its symmetric stretching frequency (C(12)–O(15) exhibits 4 cm−1 while O(15)–H(35) reveals 30 cm−1 shift).
3.5 HOMO–LUMO and density of states (DOS) studies
The calculated frontier molecular orbital (FMO) energies of CO2 closely matches the reported value obtained at B3LYP/6-31+G* level.48 The band gap (Eb) is calculated by using eqn (4) and the FMO energies are reported in Table 5.| Band gap (Eb) = ELUMO − EHOMO | (4) |
On analysing the FMO's of the adsorbents, the HOMO of PS-imidazole is the most stable followed by PS-DMA > PS-DEA and least is for PS-piperazine. Further, the HOMO's of all the adsorbents are more stable as compared to the LUMO of CO2. Since CO2 is electrophilic in nature, the HOMO of the adsorbent reacts with the LUMO of CO2 molecule.49
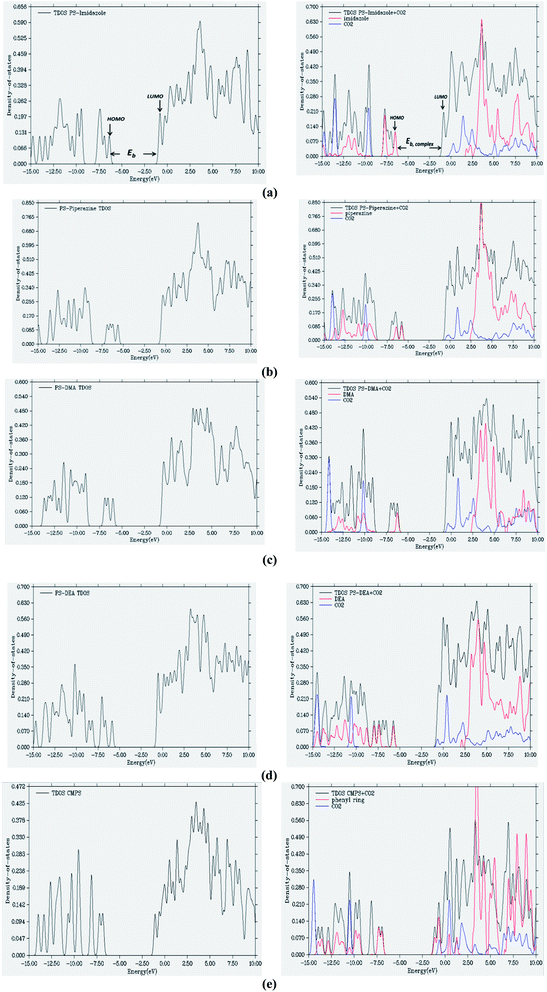
The molecules which exhibit a large HOMO–LUMO gap are considered as hard molecules and are relatively more stable than soft molecules which have a small HOMO–LUMO gap.50 To understand the interactions taking place during the adsorption of CO2, the total and partial density of states (TDOS and PDOS, respectively) of the CO2–adsorbent systems were determined and were compared with the TDOS of adsorbents without CO2 (Fig. 3). During the interaction of CO2 with the functionalized adsorbent, the electronegative donor atoms of the adsorbent attract the electron deficient ‘C’ of CO2 increasing the band gap which leads to stabilization of system, except in the case of PS–DEA–CO2 complex (Table 5). For the PS–imidazole–CO2 complex (Fig. 3(a)), the red curve (PDOS of imidaole fragment) is high and nearly approaches the black line (TDOS of complex) in the region of −6.8 to −6.1 eV. Hence it can be concluded that imidazole moiety has significant contribution towards HOMO. In contrast, the LUMO is mostly localized on the styrene moiety as there is no such clear contribution of imidazole or CO2 fragments found in the PDOS diagram. However, for the remaining amine functionalized adsorbents, the % contribution of amine towards HOMO decreases indicating lesser overlap between the amine and electron deficient ‘C’ of CO2. The PDOS of CO2 in the LUMO for PS-piperazine (Fig. 3(b)) occurs at slightly higher energy than PS-DEA (Fig. 3(d)), PS-DMA (Fig. 3(c)) and CMPS (Fig. 3(e)). However, the difference is very small (0.01–0.02 eV). The HOMO's of the adsorbent–CO2 complexes are shown in the Fig. 4.
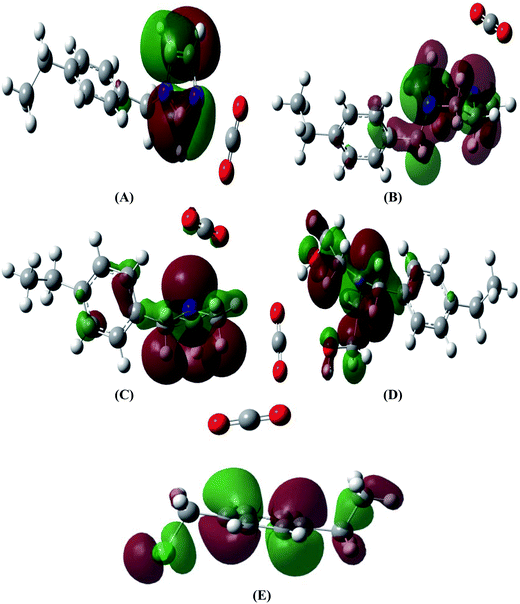 | ||
| Fig. 4 HOMO's of CO2 with PS-imidazole (A), PS-piperazine (B), PS-DMA (C), PS-DEA (D), and CMPS (E) complexes. | ||
The stability of the complex is measured in terms of %ΔEg which is defined by eqn (5)
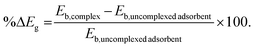 | (5) |
The values of Eb,complex and %ΔEg are reported in Table 5. As the band gap Eb,complex increases after complexation, the ability of the adsorbent to attract CO2 increases, which indicates increased stability of the system.40 The above discussion is in trend with the values of %ΔEg which is highest for PS-imidazole and lowest for PS-DEA. Since the HOMO of all the complexes presents no contribution from interacting CO2 molecule, the interactions are considered as physisorption process.
The current work involves the use of specific amines which are loaded on polystyrene and are highly selective in its interaction with CO2. The quadrupole moment of CO2 (4.3 × 10−31 C m2) induces strong and specific Lewis acid–base type interactions with the functionalized adsorbents.8 In these complexes, the ‘C’ of CO2 behaves as an electron acceptor and the ‘N or O’ of the amine as the donor. The above studies indicate PS-imidazole to be a superior adsorbent for complexing with CO2 possessing ΔE of −11.85 kJ mol−1 and shortest CCO2–Namine distance (2.8533 Å). The objective of the current study was to understand the interaction and binding of CO2 with amine functional group at the molecular level. The above study highlights the molecular level information about charge transfer, FMO contribution and vibrational frequency analysis occurring during complexation. The results indicate that the CO2–amine interaction is mainly of physisorption augmented by hydrogen bond type chemical interactions. As a result, the CO2 is adsorbed reversibly on these adsorbents and its desorption can be facilitated by thermal/pressure swing for regeneration as studied by Khot et al.8 This study aims at understanding the interaction and binding types of CO2 with amine functionalized adsorbents which can provide useful guidance for the designing of newer ligands for CO2 selective adsorption.
4 Conclusions
We investigated the interaction of CO2 on amine functionalized adsorbents containing tertiary nitrogen at the DFT level. The electrostatic interactions were responsible for the complexation of CO2 with the adsorbents. PS-imidazole shows the strongest interaction with CO2 (ΔE = −11.85 kJ mol−1) followed by PS-piperazine > PS-DMA and least for PS-DEA as evident from the interaction energies and distance between CCO2–donor atom of the adsorbent (d1). H-bonding between –OH groups of DEA and ‘O’ of CO2 occurs as ‘N’ atom of DEA is sterically hindered. During complexation, there is considerable elongation of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond closer to the interacting center while the C
O bond closer to the interacting center while the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O farthest from the donor atom of the adsorbent experiences shortening. The calculated vibrational frequency values clearly indicate that CO2 is slightly distorted from its linear shape in the complex and these calculated frequencies exhibit good correlation with experimental values. Further the analysis of thermodynamical parameters, HOMO–LUMO and DOS studies suggest weak interaction between CO2 and adsorbents indicating physisorption process. These simulation studies involving parameters of interaction energy, partial charges and vibrational spectral analysis suggest the use of imidazole loaded polystyrene as a suitable adsorbent selective for CO2 capture. The above molecular level studies help in designing of newer moieties for efficient CO2 capture systems.
O farthest from the donor atom of the adsorbent experiences shortening. The calculated vibrational frequency values clearly indicate that CO2 is slightly distorted from its linear shape in the complex and these calculated frequencies exhibit good correlation with experimental values. Further the analysis of thermodynamical parameters, HOMO–LUMO and DOS studies suggest weak interaction between CO2 and adsorbents indicating physisorption process. These simulation studies involving parameters of interaction energy, partial charges and vibrational spectral analysis suggest the use of imidazole loaded polystyrene as a suitable adsorbent selective for CO2 capture. The above molecular level studies help in designing of newer moieties for efficient CO2 capture systems.
Acknowledgements
We acknowledge the financial support for computational amenities provided by Indo-EU DST-AMCOS (Dept. of Science and Technology-Advanced Material for Computational Studies) project.References
- C. Cazorla, S. A. Shevlin and Z. X. Guo, J. Phys. Chem. C, 2011, 115, 10990 CAS.
- J. C. Hicks, J. H. Drese, D. J. Fauth, M. L. Gray, G. Qi and C. W. Jones, J. Am. Chem. Soc., 2008, 130, 2902 CrossRef CAS PubMed.
- J. J. Chen, W. W. Li, X. L. Li and H. Q. Yu, Phys. Chem. Chem. Phys., 2012, 14, 4589 RSC.
- C. M. Teague, S. Dai and D. Jiang, J. Phys. Chem. A, 2010, 114, 11761 CrossRef CAS PubMed.
- H. Yamada, Y. Matsuzaki, F. Chowdhury and T. Higashii, J. Mol. Model, 2013, 19, 4147 CrossRef CAS PubMed.
- A. B. Rao and E. S. Rubin, Environ. Sci. Technol., 2002, 36, 4467 CrossRef CAS.
- B. Arstad, H. Fjellvåg, K. O. Kongshaug, O. Swang and R. Blom, Adsorption, 2008, 14, 755 CrossRef CAS.
- K. M. Khot, P. K. Heer, R. Biniwale and V. G. Gaikar, Sep. Sci. Technol., 2014 DOI:10.1080/01496395.2014.918145.
- B. Bonelli, B. Civalleri, B. Fubini, P. Ugliengo, C. O. Areán and E. Garrone, J. Phys. Chem. B, 2000, 104, 10978 CrossRef CAS.
- W. Morris, B. Leung, H. Furukawa, O. K. Yaghi, N. He, H. Hayashi, Y. Houndonougbo, M. Asta, B. B. Laird and O. M. Yaghi, J. Am. Chem. Soc., 2010, 132, 11006 CrossRef CAS PubMed.
- L. Valenzano, B. Civalleri, S. Chavan, G. T. Palomino, C. O. Areán and S. Bordiga, J. Phys. Chem. C, 2010, 114, 11185 CAS.
- M. T. Baei, A. A. Peyghan and Z. Bagheri, Bull. Korean Chem. Soc., 2012, 33, 3338 CrossRef CAS.
- F. Akhtar, Q. Liu, N. Hedin and L. Bergstrom, Energy Environ. Sci., 2012, 5, 7664 CAS.
- R. Dawson, A. I. Cooper and D. Adams, Polym. Int., 2013, 62, 345 CrossRef CAS.
- C. Lu, H. Bai, B. Wu, F. Su and J. F. Hwang, Energy Fuels, 2008, 22, 3050 CrossRef CAS.
- M. G. Plaza, C. Pevida, A. Arenillas, F. Rubiera and J. J. Pis, Fuels, 2007, 86, 2204 CrossRef CAS PubMed.
- A. Houshmand, M. S. Shafeeyan, A. Arami-Niya and W. M. A. W. J. Daud, J. Taiwan Inst. Chem. Eng., 2013, 44, 774 CrossRef CAS PubMed.
- A. Pulido, M. R. Delgado, O. Bludský, M. Rubeš, P. Nachtigall and C. O. Areán, Energy Environ. Sci., 2009, 2, 1187 CAS.
- T. Panda, P. Pachfule, Y. Chen, J. Jiang and R. Banerjee, Chem. Commun., 2011, 47, 2011 RSC.
- F. Zheng, D. N. Tran, B. J. Busche, G. E. Fryxell, R. S. Addleman, T. S. Zemanian and C. L. Aardah, Ind. Eng. Chem. Res., 2005, 44, 3099 CrossRef CAS.
- M. Matsuguchi, N. Maeda and Y. Sakai, J. Appl. Polym. Sci., 2002, 83, 401 CrossRef CAS.
- T. Filburn, J. J. Helble and R. A. Weiss, Ind. Eng. Chem. Res., 2005, 44, 1542 CrossRef CAS.
- A. Diaf, J. L. Garcia and E. J. Beckman, J. Appl. Polym. Sci., 1994, 53, 857 CrossRef CAS.
- W. R. Alesi, Jr. and J. R. Kitchin, Ind. Eng. Chem. Res., 2012, 51, 6907 CrossRef.
- S. G. Kazarian, M. F. Vincent, F. V. Bright, C. L. Liotta and C. A. Eckert, J. Am. Chem. Soc., 1996, 118, 1729 CrossRef CAS.
- N. Hiyoshi, K. Yogo and T. Yashima, Microporous Mesoporous Mater., 2005, 84, 357 CrossRef CAS PubMed.
- G. P. Knowles, S. W. Delaney and A. L. Chaffee, Ind. Eng. Chem. Res., 2006, 45, 2626 CrossRef CAS.
- A. Sayari, Y. Belmabkhout and E. Da'na, Langmuir, 2012, 28, 4241 CrossRef CAS PubMed.
- D. P. Thakur, N. P. Barde, P. P. Bardapurkar and R. S. Khairnar, Ukr. J. Phys., 2013, 58(9), 841 CAS.
- M. A. Hussain, Y. Soujanya and G. N. Sastry, Environ. Sci. Technol., 2011, 45, 8582 CrossRef CAS PubMed.
- Y. Xie, T. T. Wang, X. H. Liu, K. Zou and W. Q. Deng, Nat. Commun., 2013, 4, 1960 Search PubMed.
- Y. Kuwahara, D. Y. Kang, J. R. Copeland, P. Bollini, C. Sievers, T. Kamegawa, H. Yamashita and C. W. Jones, Chem. Eur. J., 2012, 18, 16649 CrossRef CAS PubMed.
- H. A. Patel, F. Karadas, A. Canlier, J. Park, E. Deniz, Y. Jung, M. Atilhan and C. T. Yavuz, J. Mater. Chem., 2012, 22, 8431 RSC.
- P. Muchana, C. Saiwana, D. de Montigny and P. Tontiwachwuthikulb, Chemical Engineering Transactions, 2013, 35, 391 Search PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, GAUSSIAN 09 (Revision B.01), Gaussian Inc., Wallingford CT, 2009 Search PubMed.
- J. Baltrusaitis and V. H. Grassian, J. Phys. Chem. A, 2010, 114, 2350 CrossRef CAS PubMed.
- H. Kayi, R. I. Kaiserab and J. D. Head, Phys. Chem. Chem. Phys., 2011, 13, 11083 RSC.
- T. Lu and F. Chen, J. Comp. Chem., 2012, 33, 580 CrossRef CAS PubMed.
- R. J. Xavier and P. Dinesh, Spectrochim. Acta, Part A, 2013, 113, 171 CrossRef CAS PubMed.
- A. A. Rafati, S. M. Hashemianzadeh and Z. B. Nojini, J. Phys. Chem. C, 2008, 112, 3597 CAS.
- K. M. Khot, P. K. Heer, R. Biniwale and V. G. Gaikar, Sep. Sci. Technol. Search PubMed , submitted.
- Y. Danten, T. Tassaing and M. Besnard, J. Phys. Chem. A, 2002, 106, 11831 CrossRef CAS.
- J. G. Vitillo, M. Savonnet, G. Ricchiardi and S. Bordiga, ChemSusChem., 2011, 4, 1281 CrossRef CAS PubMed.
- M. Hargittai and I. Hargittai, Int. J. Quantum Chem., 1992, 44, 1057 CrossRef CAS.
- A. R. Patil, J. S. Arora and V. G. Gaikar, Sep. Sci. Technol., 2012, 47, 1156 CrossRef CAS.
- Y. Yao, N. Nijem, J. Li, Y. J. Chabal, D. C. Langreth and T. Thonhauser, Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 85, 1 Search PubMed.
- Y. Liu, S. Zhao, X. Dong, K. Yuan, H. Tang, G. Zuo, Y. Zhu and X. Liu, Journal of Atomic and Molecular Sciences, 2011, 2, 234 CrossRef.
- N. Kuş, S. Breda, I. Reva, E. Tasal, C. Ogretir and R. Fausto, Photochem. Photobiol., 2007, 83, 1237 CrossRef PubMed.
- H. Wu, J. M. Simmons, G. Srinivas, W. Zhou and T. J. Yildirim, J. Phys. Chem. Lett., 2010, 1, 1946 CrossRef CAS.
- R. G. Pearson, Proc. Natl. Acad. Sci. U. S. A., 1986, 83, 8440 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |