Dopamine polymerization-induced surface colouration of various materials
Abstract
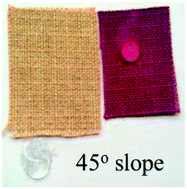
Bio-inspired by melanins and adhesive of marine mussels, a novel method was developed for colouration of various material surfaces. Using dopamine polymerization to form an adhesive coating, the surface colouration of various materials was easily achieved, including metal, ceramic, polymers and even textile fabrics (resistant to colouration) through a simple dip-coating procedure. The colour appearance of the dyed materials could be tuned in a controllable way due to the reactivity of dopamine with nucleophiles such as amino acids and heterocycle components during its oxidization step. Commercially available colorants could also be used in this procedure to enrich the colour gamut. The surface compositions, morphology and wettability of the dyed surfaces were studied by X-ray photoelectron spectroscopy, scanning electron microscopy and water contact angle measurement, respectively. The obtained results showed that the material surfaces were successfully coloured, which was verified by the obvious changes in surface properties compared to the blank samples. For this colouration method, less energy consumption and dyeing auxiliaries were needed, indicating that it is an environmentally friendly approach. Moreover, it is a promising alternative to the traditional colouration techniques, especially for those materials, which are resistant to colouration.

 Please wait while we load your content...
Please wait while we load your content...