Sensitive and regenerable organochalcogen probes for the colorimetric detection of thiols†
Shah Jaimin Balkrishna,
Ananda S. Hodage,
Shailesh Kumar,
Piyush Panini and
Sangit Kumar*
Department of Chemistry, Indian Institute of Science Education and Research (IISER), Bhopal, Madhya Pradesh 462066, India. E-mail: sangitkumar@iiserb.ac.in; Tel: +91-755-6692326
First published on 12th February 2014
Abstract
Isothiazolone and isoselenazolone based colorimetric probes have been reported for the detection of thiols. The isothiazolone probe detected two equiv. of thiols. A regenerable probe is developed from isoselenazolone. Both probes possess high selectivity for aromatic thiols, cysteine and glutathione.
Rapid, sensitive and selective detection of biologically active and toxic molecules is of significant importance in the fields of chemical, biological and environmental sciences.1 Compounds with thiol (–SH) functionality are very important as low molecular weight aliphatic thiol containing amino acids (cysteine)2a and peptides (glutathione)2b,c play important roles in biological systems while aromatic thiols (thiophenols)2d are important reagents and possess broad synthetic utility. In spite of their application as important building blocks in organic synthesis, aromatic thiols are considered as toxic and pollutant compounds.3
Recently, significant efforts have been made for the development of probes capable of detecting thiols such as cysteine (Cys), glutathione (GSH) and thiophenols.4,5 Most of these probe molecules react with thiol functionality to form a covalent bond via irreversible reaction and possess probe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) thiol detection ratio of 1
thiol detection ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Therefore, a probe which can detect more than one equiv. of thiol and a probe which can be easily recycled are highly desirable.
1. Therefore, a probe which can detect more than one equiv. of thiol and a probe which can be easily recycled are highly desirable.
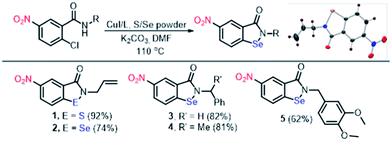
Isothiazolone and selenazolones are a class of organochalcogen compounds and possess many biological activities.6 One of the isoselenazolone; ebselen is biologically non-toxic and decomposes hydroperoxides catalytically utilizing organothiol substrates. In continuation of our work on organochalcogen chemistry,7 we report new and mechanistically different low molecular weight organochalcogen colorimetric probes with the following salient features; (a) high specificity of both probes 1 and 2 for thiophenols, cysteine and glutathione, (b) rapid response (colorless to bright yellow), (c) detection of two equiv. thiophenols by isothiazolone probe 1, (d) regenerability of isoselenazolone probe 2 for >10 cycles. Isothiazolone 1 and isoselenazolones 2–5 were synthesized from corresponding 2-chlorobenzamides by Cu-catalyzed thiolation/selenation reaction.6 The presence of S–N and Se–N bonds in 1–4 is also established by single crystal X-ray studies (Scheme 1).
Isothiazolone 1 (100 μM) absorbs at 336 nm (Fig. 1 and 2). With increasing concentration of PhSH, peak at 336 nm due to 1 decreased gradually with appearance of a new peak at 413 nm and immediate yellow color appearance was noticed. The stoichiometric ratio between probe 1 and PhSH was observed as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 based on the change of absorbance at 413 nm which suggest that the probe 1 can detect up to 2 equiv. of PhSH. Next various aromatic and aliphatic thiols; ethane-, n-hexane-, tert-butyl-thiols, benzyl thiol, 2-mercapto pyridine, 2-amino-, 2-methoxy-, 4-methoxy-, 4-methyl-, 4-chloro-thiophenols, biologically important organic molecules; glucose, L-proline, ascorbic acid, glycine, alanine, arginine, GSH, cysteine, N-acetyl-L-cysteine, DTTred and nucleophiles like aniline, 4-methoxy phenol were investigated to study selectivity of probe 1 towards these substrates (see Fig. S39 in ESI†). Probe 1 exhibited excellent selectivity towards thiophenols, cysteine, glutathione and dithiothreitol compared to the remainder of the substrates. Next, the effect of various metal ions on the performance of probe 1 was studied by preparing solution containing probe 1 (100 μM) and the metal ion (200 μM). Salts of Na+, K+, Cs+, Mg2+, Ca2+, Zn2+, Pd2+, Ba2+, Ni2+, Co2+, Al3+ Cr3+, Cd2+ didn't interfere in the analysis as the yellow color remained unaffected and also UV-visible absorption spectra were unchanged. On the other hand, Cu2+, Hg2+ and Ag+ inhibited activity of probe 1 completely presumably due to their ability to form coordination complexes with PhSH. For these metals, equimolar (200 μM) solution of chelating EDTA ligand was added to shield them and probe 1 regained its ability to sense the thiol colorimetrically (page S50–S52 in ESI†).
2 based on the change of absorbance at 413 nm which suggest that the probe 1 can detect up to 2 equiv. of PhSH. Next various aromatic and aliphatic thiols; ethane-, n-hexane-, tert-butyl-thiols, benzyl thiol, 2-mercapto pyridine, 2-amino-, 2-methoxy-, 4-methoxy-, 4-methyl-, 4-chloro-thiophenols, biologically important organic molecules; glucose, L-proline, ascorbic acid, glycine, alanine, arginine, GSH, cysteine, N-acetyl-L-cysteine, DTTred and nucleophiles like aniline, 4-methoxy phenol were investigated to study selectivity of probe 1 towards these substrates (see Fig. S39 in ESI†). Probe 1 exhibited excellent selectivity towards thiophenols, cysteine, glutathione and dithiothreitol compared to the remainder of the substrates. Next, the effect of various metal ions on the performance of probe 1 was studied by preparing solution containing probe 1 (100 μM) and the metal ion (200 μM). Salts of Na+, K+, Cs+, Mg2+, Ca2+, Zn2+, Pd2+, Ba2+, Ni2+, Co2+, Al3+ Cr3+, Cd2+ didn't interfere in the analysis as the yellow color remained unaffected and also UV-visible absorption spectra were unchanged. On the other hand, Cu2+, Hg2+ and Ag+ inhibited activity of probe 1 completely presumably due to their ability to form coordination complexes with PhSH. For these metals, equimolar (200 μM) solution of chelating EDTA ligand was added to shield them and probe 1 regained its ability to sense the thiol colorimetrically (page S50–S52 in ESI†).
Fig. 3a describes the change in the UV spectrum of probe 2 (100 μM) when the PhSH solution is added to it in PBS buffer (10 mM, pH 7.4)–acetonitrile (75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25). Upon gradual addition of one equiv. of PhSH, absorption peak of 2 at 349 nm is slightly blue shifted to 343 nm without any colour change. This peak at 343 nm is gradually decreased with an emergence of a new peak at 426 nm upon gradual addition of one more equivalent of PhSH. A rapid visual colour change is observed from colourless to bright yellow in solution. Absorbance at 426 nm as a function of PhSH concentration indicates 1
25). Upon gradual addition of one equiv. of PhSH, absorption peak of 2 at 349 nm is slightly blue shifted to 343 nm without any colour change. This peak at 343 nm is gradually decreased with an emergence of a new peak at 426 nm upon gradual addition of one more equivalent of PhSH. A rapid visual colour change is observed from colourless to bright yellow in solution. Absorbance at 426 nm as a function of PhSH concentration indicates 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio for PhSH and probe 2 (inset of Fig. 3a). Probe 2 displayed similar selectivity towards aliphatic and aromatic thiols, biomolecules and also similar interference by metal salts as the case with probe 1. However, in addition to Cu2+, Hg2+ and Ag+ ions, Cd2+ ion also inhibited the sensing property of isoselenazolone 2 (see Fig. S44 and S45 in ESI†). Isoselenazolone functions as a catalyst using thiol substrates for the reduction of hydroperoxides. To see the reusability of probes in the sensing activity, yellow coloured solution of isothiazolone 1 and PhSH was treated with excess of TBHP. Unfortunately, regeneration of probe 1 was not noticed as absorption spectra of the solution remained unshifted. Next, isoselenazolones 2–5 were tested for regenerability. To the yellow solution of probe 2 and PhSH, excess of TBHP (5 equiv.) was mixed and allowed to react for a minute. It resulted into colourless solution and UV spectrum was again blue shifted to 343 nm. Again PhSH (2 equiv.) was added to this colourless solution and it resulted into red-shift to 426 nm in the absorption spectra. Reversibility of probe 2 was evaluated for 11 cycles by alternative addition of oxidant TBHP and reductant PhSH (Fig. 3b). Other isoselenazolone derivatives 3–5 possessing structural similarities with probe 2 were also studied for regenerable thiol detection. Isoselenazolone 3–5 showed similar results as probe 2 but with poor regenerability as precipitation was observed after two cycles. Next we examined detection limits for probes 1 and 2 and they are found sensitive towards the detection of thiol up to 2 μM and 10 μM respectively (see Fig. S50 in ESI†).
1 ratio for PhSH and probe 2 (inset of Fig. 3a). Probe 2 displayed similar selectivity towards aliphatic and aromatic thiols, biomolecules and also similar interference by metal salts as the case with probe 1. However, in addition to Cu2+, Hg2+ and Ag+ ions, Cd2+ ion also inhibited the sensing property of isoselenazolone 2 (see Fig. S44 and S45 in ESI†). Isoselenazolone functions as a catalyst using thiol substrates for the reduction of hydroperoxides. To see the reusability of probes in the sensing activity, yellow coloured solution of isothiazolone 1 and PhSH was treated with excess of TBHP. Unfortunately, regeneration of probe 1 was not noticed as absorption spectra of the solution remained unshifted. Next, isoselenazolones 2–5 were tested for regenerability. To the yellow solution of probe 2 and PhSH, excess of TBHP (5 equiv.) was mixed and allowed to react for a minute. It resulted into colourless solution and UV spectrum was again blue shifted to 343 nm. Again PhSH (2 equiv.) was added to this colourless solution and it resulted into red-shift to 426 nm in the absorption spectra. Reversibility of probe 2 was evaluated for 11 cycles by alternative addition of oxidant TBHP and reductant PhSH (Fig. 3b). Other isoselenazolone derivatives 3–5 possessing structural similarities with probe 2 were also studied for regenerable thiol detection. Isoselenazolone 3–5 showed similar results as probe 2 but with poor regenerability as precipitation was observed after two cycles. Next we examined detection limits for probes 1 and 2 and they are found sensitive towards the detection of thiol up to 2 μM and 10 μM respectively (see Fig. S50 in ESI†).
We were interested in detecting species responsible for the color change upon addition of PhSH to probe 1. Therefore, a stoichiometric reaction was carried out between probe 1 and PhSH in PBS buffer–acetonitrile (75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25) at room temperature followed by isolation of compound, which confirmed to be 1b after characterization (Scheme 2). UV spectrum on isolated 1b (λmax = 413 nm) provided further confirmation. We believe that probe 1 forms unsymmetrical disulfide 1a with one equiv. of PhSH. Disulfide 1a seems to be transient species (detected by mass spectrometry only) and converts immediately into coloured thiol 1b. This could correlate the unprecedented detection of two equiv. of thiophenol.
25) at room temperature followed by isolation of compound, which confirmed to be 1b after characterization (Scheme 2). UV spectrum on isolated 1b (λmax = 413 nm) provided further confirmation. We believe that probe 1 forms unsymmetrical disulfide 1a with one equiv. of PhSH. Disulfide 1a seems to be transient species (detected by mass spectrometry only) and converts immediately into coloured thiol 1b. This could correlate the unprecedented detection of two equiv. of thiophenol.
For mechanistic insight, probe 2 was reacted with one equiv. of PhSH under identical experimental conditions and isolated product was confirmed as selenenylsulfide 2a. UV absorption spectra of isolated 2a gave λmax at 343 nm. To the solution of 2a, an equiv. of PhSH was added, which resulted in the red shift to 426 nm with appearance of bright yellow colour. The sample was subjected for mass analysis and noticed to be selenol 2b (m/z = 284.9). Worth noticing, aqueous medium is important for the generation of 2b from 2a because 2a failed to show any color change and also change in the UV absorption spectrum upon the addition of one equiv. of PhSH in CH3OH/CH3CN medium.
Therefore, aqueous medium was necessary for generation of selenol 2b which is responsible for sensing activity. The unprecedented solvent dependent generation of selenol 2b from 2a was further validated by reacting in situ generated 2b with CH3I. Complete conversion of selenol 2b into methyl selenide 2d was observed, which was isolated and characterized. Interestingly, reaction in CH3OH failed to provide any 2d. TBHP was added to in situ generated selenol 2b to understand the intermediate involved in the regenerability and solution was analysed by mass spectrometry, which showed the molecular ion peak for selenenic acid 2c (m/z = 300.9). Thus regenerable mode of probe 2 is presented in Scheme 3 based on mass analysis and control experiments.
 | ||
| Scheme 3 Mechanistic pathway for reversible nature of probe 2 and trapping of key intermediate selenol 2b by quenching it with CH3I. | ||
Conclusions
In summary, we have developed two low molecular weight organochalcogen probes for the colorimetric detection of thiols which work well in an aqueous neutral (pH 7.4) medium with instantaneous response. Both probes showed selectivity for aromatic thiols, cysteine and glutathione. Isothiazolone probe efficiently detects two equiv. of thiol following irreversible pathway, while isoselenazolone probe detected in a regenerable manner for >10 cycles. Important character of probe 2 is that the detection of thiols can be conducted in a reversible manner by simple visual inspection without the use of any expensive instrument. The species, responsible for the color change are also characterized. The formation of benzamide derived thiol and selenol in the aqueous medium is responsible for characteristic color change.Acknowledgements
SJB, ASH, SK thank IISER Bhopal for fellowships and SK thanks DST, DRDO-New Delhi, DAE-Mumbai for financial support to this work.Notes and references
- For recent reviews on optical sensors for thiols, see: (a) X. Chen, Y. Zhou, X. Peng and J. Yoon, Chem. Soc. Rev., 2010, 39, 2120 RSC; (b) Y. Zhou and J. Yoon, Chem. Soc. Rev., 2012, 41, 52 RSC; (c) J. Chan, S. C. Dodani and C. J. Chang, Nat. Chem., 2012, 4, 973 CrossRef CAS PubMed; (d) H. Peng, W. Chen, Y. Cheng, L. Hakuna, R. Strongin and B. Wang, Sensors, 2012, 12, 15907 CrossRef CAS PubMed; (e) C. Yin, F. Huo, J. Zhang, R. M. Máñez, Y. Yang, H. Lv and S. Li, Chem. Soc. Rev., 2013, 42, 6032 RSC and references there in.
- (a) A. Fersht, Enzyme Structure and Mechanism, Freeman, Co., New York, 2nd edn, 1984, pp. 2–4 Search PubMed; (b) C.-S. Hwang, A. J. Sinskey and H. F. Lodish, Science, 1992, 257, 1496 CAS; (c) R. Franco, M. I. Panayiotidis and J. A. Cidcowski, J. Biol. Chem., 2007, 282, 30452 CrossRef CAS PubMed; (d) J. C. Love, L. A. Estroff, J. K. Kriebel, R. G. Nuzzo and G. M. Whitesides, Chem. Rev., 2005, 105, 1103 CrossRef CAS PubMed.
- (a) Material safety data sheet of thiophenol from Sigma-Aldrich, http://www.castleviewuk.com/Frameless/Safe/msds/ex/MSDS_thiophenol.htm; (b) Health Council of the Netherlands, Committee on Updating of Occupational Exposure Limits. Benzenethiol: Health-based Reassessment of Administrative Occupational Exposure Limits, The Hague: Health Council of the Netherlands, 2004, 2000/15OSH/095; (c) Anon, USA, Dangerous Prop. Ind. Mater. Rep., 1994, 14, 92 Search PubMed; (d) Interim acute exposure guideline levels (AEGLs) for phenyl mercaptan (C6H5SH), Interim 1: 11/2007 US Environmental Protection Agency; (e) EPA Method 8270C, Semivolatile Organic Compounds by Gas Chromatography/Mass Spectrometry (GC/MS), US Environmental Protection Agency, December, 1996.
- Recent references of organochalcogen probes: (a) B. Tang, Y. Xing, P. Li, N. Zhang, F. Yu and G. Yang, J. Am. Chem. Soc., 2007, 129, 11666 CrossRef CAS PubMed; (b) B. Tang, L. Yin, X. Wang, Z. Chen, L. Tong and K. Xu, Chem. Commun., 2009, 5293 RSC; (c) Z. Chen, Q. Li, X. Wang, Z. Wang, R. Zhang, M. Yin, L. Yin, K. Xu and B. Tang, Anal. Chem., 2010, 82, 2006 CrossRef CAS PubMed; (d) R. Wang, L. Chen, P. Liu, Q. Zhang and Y. Wang, Chem. –Eur. J., 2012, 18, 11343 CrossRef CAS PubMed; (e) K. Xu, M. Qiang, W. Gao, R. Su, N. Li, Y. Gao, Y. Xie, F. Kong and B. Tang, Chem. Sci., 2013, 4, 1079 RSC; (f) D. P. Murale, S. T. Manjare, Y.-S. Lee and D. G. Churchill, Chem. Commun., 2014, 50, 359 RSC; (g) S. T. Manjare, S. Kim, W. D. Heo and D. G. Churchill, Org. Lett., 2014, 16, 410–412 CrossRef CAS PubMed; (h) S. T. Manjare, J. Kim, Y. Lee and D. G. Churchill, Org. Lett., 2014, 16, 520–523 CrossRef CAS PubMed.
- (a) O. Rusin, N. S. Luce, R. A. Agbaria, J. O. S. Escobedo, I. Jiang, M. Warner, F. B. Dawan, K. Lian and R. M. Strongin, J. Am. Chem. Soc., 2004, 126, 438 CrossRef CAS PubMed; (b) W. H. Wang, J. O. Escobedo, C. M. Lawence and R. M. Strongin, J. Am. Chem. Soc., 2004, 126, 3400 CrossRef CAS PubMed; (c) N. Shao, J. Y. Jin, S. M. Cheung, R. H. Yang, W. H. Chan and T. Mo, Angew. Chem., Int. Ed., 2006, 45, 4944 CrossRef CAS PubMed; (d) Y. Zeng, G. Zhang and D. Zhang, Anal. Chim. Acta, 2008, 627, 254 CrossRef CAS PubMed; (e) L. Caroen and J. V. D. Eycken, Tetrahedron Lett., 2009, 50, 41 CrossRef PubMed; (f) S. J. Yang, X. Z. Tian and I. Shin, Org. Lett., 2009, 11, 3438 CrossRef CAS PubMed; (g) F.-J. Huo, Y.-Q. Sun, J. Su, J.-B. Chao, H.-J. Zhi and C.-X. Yin, Org. Lett., 2009, 11, 4918 CrossRef CAS PubMed; (h) X. Chen, S.-K. Ko, M. J. Kim, I. Shin and J. Yoon, Chem. Commun., 2010, 46, 2751 RSC; (i) Z. Guo, S. Nam, S. Park and J. Yoon, Chem. Sci., 2012, 3, 2760 RSC; (j) L. Deng, W. Hu, H. Guo, J. Zhao, S. Ji, X. Zhang, X. Yuan and C. Zhang, J. Org. Chem., 2011, 76, 9294 CrossRef CAS PubMed; (k) M. Cacciarini, E. A. Della Pia and M. B. Nielsen, Eur. J. Org. Chem., 2012, 6064 CrossRef CAS; (l) D. Kand, P. K. Mishra, T. Saha, M. Lahiri and P. Talukdar, Analyst, 2012, 137, 3921 RSC; (m) D. Kand, A. M. Kalle, S. J. Varma and P. Talukdar, Chem. Commun., 2012, 48, 2722 RSC; (n) D. Kand, A. M. Kalle and P. Talukdar, Org. Biomol. Chem., 2013, 11, 1691 RSC; (o) W. Jiang, Q. Fu, H. Fan, J. Ho and W. Wang, Angew. Chem., Int. Ed., 2007, 46, 8445 CrossRef CAS PubMed; (p) W. Jiang, Y. Cao, Y. Liu and W. Wang, Chem. Commun., 2010, 46, 1944 RSC; (q) D. P. Murale, H. Kim, W. S. Choi and D. G. Churchill, Org. Lett., 2013, 15, 3630 CrossRef CAS PubMed; (r) O. G. Tsay, K. M. Lee and D. G. Churchill, New J. Chem., 2012, 36, 1949 RSC; (s) D. P. Murale, H. Kim, W. S. Choi and D. G. Churchill, RSC Adv., 2014, 4, 5289–5292 RSC.
- (a) G. Mugesh and H. B. Singh, Chem. Soc. Rev., 2000, 29, 347 RSC; (b) G. Mugesh, W.-W. DuMont and H. Sies, Chem. Rev., 2001, 101, 2125 CrossRef CAS PubMed; (c) K. P. Bhabak and G. Mugesh, Chem. –Eur. J., 2007, 13, 4594 CrossRef CAS PubMed; (d) K. P. Bhabak and G. Mugesh, Acc. Chem. Res., 2010, 43, 1408 CrossRef CAS PubMed; (e) K. Selvakumar, P. Shah, H. B. Singh and R. J. Butcher, Chem. –Eur. J., 2011, 17, 12741 CrossRef CAS PubMed.
- (a) S. J. Balkrishna, B. S. Bhakuni, D. Chopra and S. Kumar, Org. Lett., 2010, 12, 5394 CrossRef CAS PubMed; (b) S. J. Balkrishna, B. S. Bhakuni and S. Kumar, Tetrahedron, 2011, 67, 9565 CrossRef CAS PubMed; (c) B. S. Bhakuni, S. J. Balkrishna, A. Kumar and S. Kumar, Tetrahedron Lett., 2012, 53, 1354 CrossRef CAS PubMed; (d) S. J. Balkrishna, C. D. Prasad, P. Panini, M. R. Detty, D. Chopra and S. Kumar, J. Org. Chem., 2012, 77, 9541 CrossRef CAS PubMed; (e) S. J. Balkrishna, S. Kumar, G. K. Azad, B. S. Bhakuni, P. Panini, N. Ahalawat, R. S. Tomar, M. R. Detty and S. Kumar, Org. Biomol. Chem., 2014, 12, 1215–1219 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, spectroscopic data. Crystal structures for 1–4 [CCDC no. 978032, 978031, 953727, 978033, respectively]. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ra00381k |
| This journal is © The Royal Society of Chemistry 2014 |