Sensitive and regenerable organochalcogen probes for the colorimetric detection of thiols†
Abstract
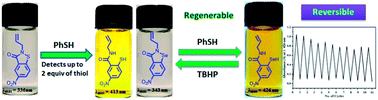
Isothiazolone and isoselenazolone based colorimetric probes have been reported for the detection of thiols. The isothiazolone probe detected two equiv. of thiols. A regenerable probe is developed from isoselenazolone. Both probes possess high selectivity for aromatic thiols, cysteine and glutathione.

 Please wait while we load your content...
Please wait while we load your content...