Recent advances in 4(3H)-quinazolinone syntheses
Abstract
The 4(3H)-quinazolinone is a frequently encountered heterocycle with broad applications including antimalarial, antitumor, anticonvulsant, fungicidal, antimicrobial, and anti-inflammatory. The current review article will briefly outline the new routes and strategies for the synthesis of valuable 4(3H)-quinazolinones.
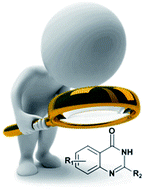
- This article is part of the themed collection: Organic chemistry collection

 Please wait while we load your content...
Please wait while we load your content...