Development of a cell permeable ratiometric chemosensor and biomarker for hydrogen sulphate ions in aqueous solution†
Buddhadeb Sena,
Manjira Mukherjeea,
Siddhartha Pala,
Sushil Kumar Mandalb,
Maninder S. Hundalc,
Anisur Rahman Khuda-Bukhshb and
Pabitra Chattopadhyay*a
aDepartment of Chemistry, The University of Burdwan, Golapbag, Burdwan 713104, India. E-mail: pabitracc@yahoo.com; Fax: +91-342-2530452; Tel: +91-342-2558554 extn 424
bMolecular Biology and Genetics Laboratory, Department of Zoology, Kalyani University, India
cDepartment of Chemistry, Guru Nanak Dev University, Amritsar 143005, India
First published on 12th March 2014
Abstract
A newly designed organic moiety, 5H-5,7a,12-triaza-dibenzo[a,e]azulen-6-one (L) containing a seven membered ring behaves as a hydrogen sulphate ion selective ratiometric chemosensor. The formulation and detailed structural characterisation of L have been established using physico-chemical, spectroscopic tools and single crystal X-ray diffraction study. On additions of hydrogen sulphate ions to the solution of L in HEPES buffer (1 mM; water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol (v/v), 98
ethanol (v/v), 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) at 25 °C at biological pH, a new fluorescence peak generated at 483 nm was increased with concomitant decrease of the weak fluorescence of L at 430 nm through an isoemissive point at 449 nm due to the selective binding of HSO4− ions with L in a 1
2) at 25 °C at biological pH, a new fluorescence peak generated at 483 nm was increased with concomitant decrease of the weak fluorescence of L at 430 nm through an isoemissive point at 449 nm due to the selective binding of HSO4− ions with L in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio with a binding constant (K) of 4.13 × 106 M−1, and detects HSO4− ions as low as 5.5 × 10−7 M. The ratiometric enhancement of the fluorescence is based on intermolecular hydrogen bonding assisted chelation enhanced fluorescence (CHEF) process which has been evidenced by 1HNMR titration and supported by theoretical (DFT) calculations. The probe (L) having no cytotoxic effect is also useful for the detection of intracellular HSO4− ion concentrations under a fluorescence microscope.
1 ratio with a binding constant (K) of 4.13 × 106 M−1, and detects HSO4− ions as low as 5.5 × 10−7 M. The ratiometric enhancement of the fluorescence is based on intermolecular hydrogen bonding assisted chelation enhanced fluorescence (CHEF) process which has been evidenced by 1HNMR titration and supported by theoretical (DFT) calculations. The probe (L) having no cytotoxic effect is also useful for the detection of intracellular HSO4− ion concentrations under a fluorescence microscope.
Introduction
Anions play a vital role in a wide range of industrial, agricultural, biological systems and environmental processes.1 Thus, the design and development of selective optical chemosensors for various anions have gained considerable attention.2 Among the various anions, hydrogen sulfate (HSO4−) ions dissociate at high pH to generate toxic sulfate (SO42−), causing irritation of the skin and eyes and even respiratory paralysis. Despite its crucial roles in biological processes, only few examples of sensors for HSO4− ions have been reported.3,4 Among all these sensors, most of them performed through single point detection3 and only few acted upon ratiometric titration,4 but none of them as a cell permeable sensor for hydrogen sulphate in aqueous solvent has been reported. However, it is well known that ratiometric responses are more attractive because the ratio between the two emission intensities can be used to measure the analyte concentration and provide a built-in correction for environmental effects and stability under illumination.5,6 So, it is a challenge for a chemist to develop a ratiometric water soluble cell permeable fluorescent probe for hydrogen sulfate ions.Chemosensors based on anion-induced changes in fluorescence are particularly attractive because of the simplicity, high spatial and temporal resolution of fluorescence.7 Secondary amides have been employed widely as hydrogen bond donor groups to bind anionic species.8 The first example of a synthetic anion receptor containing secondary amides was reported by Pascal and co-workers in 1986.9 Among the various types of these receptors, imidazolium based receptors have also been extensively investigated in recent years.
In the search of a water soluble HSO4− ions selective chemosensor, we designed and synthesized a new compound (L) in a facile route using imidazolium based secondary amides. L behaves as a ratiometric amplified chemosensor highly selective and sensitive fluorescent probe for HSO4− ions in HEPES buffer (1 mM, pH 7.4; 2% EtOH) at 25 °C upto a very low concentration (5.5 × 10−7 M) of HSO4− ions. This probe (L) was also useful to detect the presence of bisulphate ions by acquiring image of HeLa cells under a fluorescence microscope and L has no cytotoxicity. To the best of our knowledge so far, the 5H-5,7a,12-triaza-dibenzo[a,e]azulen-6-one (L) containing a seven membered ring as a water soluble cell permeable ratiometric fluorescent probe for hydrogen sulfate ions is still unexplored.3,4
Experimental section
Materials and methods
High-purity HEPES [4-(2-hydroxyethyl)-1-piperazineethane-sulfonic acid], 2-(2-aminophenyl)benz-imidazole, 2-chloroacetyl chloride, and tetra butyl ammonium hydrogen sulphate were purchased from Sigma Aldrich (India), solvents used were spectroscopic grade. Other chemicals, tetrabutyl ammonium salts of fluoride, chloride, bromide, iodide, acetate, dihydrogen phosphate and sodium salt of monohydrogen phosphate, azide, thiocyanate and nitrate were of analytical reagent grade and used without further purification except when specified. Milli-Q, 18.2 MΩ cm−1 water was used throughout all experiments. The elemental analyses (C, H and N) were performed on a Perkin Elmer 2400 CHN elemental analyzer. A Shimadzu (model UV-1800) spectrophotometer was used for recording electronic spectra. FTIR spectra were recorded using Prestige-21 SHIMADZU FTIR spectrometer preparing KBr disk. 1HNMR spectrum of organic moiety was obtained on a Bruker Avance DPX 500 MHz spectrometer using DMSO-d6 solution. Electrospray ionization (ESI) mass spectra were recorded on a Qtof Micro YA263 mass spectrometer. A Systronics digital pH meter (model 335) was used to measure the pH of the solution and the adjustment of pH was done using either 50 mM HCl or sodium hydroxide solution. Steady-state fluorescence emission and excitation spectra were recorded with a Hitachi F-4500 FL Spectrophotometer. Time-resolved fluorescence lifetime measurements were performed using a HORIBA JOBIN Yvon picosecond pulsed diode laser-based time-correlated single-photon counting (TCSPC) spectrometer from IBH (UK) at λex = 377 nm and MCP-PMT as a detector. Emission from the sample was collected at a right angle to the direction of the excitation beam maintaining magic angle polarization (54.71). The full width at half-maximum (FWHM) of the instrument response function was 250 ps, and the resolution was 28.6 ps per channel. Data were fitted to multiexponential functions after deconvolution of the instrument response function by an iterative reconvolution technique using IBH DAS 6.2 data analysis software in which reduced w2 and weighted residuals serve as parameters for goodness of fit.Synthetic procedures
L. C15H11N3O. Anal. found: C, 72.52; H, 4.39; N, 17.01; calc.: C, 72.28; H, 4.45; N, 16.86; IR (KBr, cm−1): νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, 1687, νN–H, 3205. (See ESI†); 1H NMR (400 MHz, DMSO-d6): 10.637 (s, 1H), 8.087 (dd, 1H), 7.824 (dd, 1H), 7.745 (dd, 1H), 7.574 (q, 1H), 7.377–7.266 (m, 4H), 4.946 (s, 2H, –CH2–CO); ESI-MS m/z 249.98 [M + H+, 100%]; calc.: 250.09; [M + H]+; 13C NMR (DMSO-d6): 166.332, 149.161, 137.348, 133.602, 131.465, 126.226, 126.041, 124.957, 118.106, 115.203, 46.215. Yield: 78%.
O, 1687, νN–H, 3205. (See ESI†); 1H NMR (400 MHz, DMSO-d6): 10.637 (s, 1H), 8.087 (dd, 1H), 7.824 (dd, 1H), 7.745 (dd, 1H), 7.574 (q, 1H), 7.377–7.266 (m, 4H), 4.946 (s, 2H, –CH2–CO); ESI-MS m/z 249.98 [M + H+, 100%]; calc.: 250.09; [M + H]+; 13C NMR (DMSO-d6): 166.332, 149.161, 137.348, 133.602, 131.465, 126.226, 126.041, 124.957, 118.106, 115.203, 46.215. Yield: 78%.
Compound 2. Bu4N[C15H12N3O5S]: C31H48N4O5S. Anal. found: C, 63.53; H, 8.08; N, 9.74; S, 5.19; calc.: C, 63.24; H, 8.22; N, 9.52; S, 5.45; IR (KBr, cm−1): νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, 1643, νO–H, 3422; νS
O, 1643, νO–H, 3422; νS![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O 1113. 1H NMR (400 MHz, DMSO-d6): 11.060 (s, 1H), 8.212 (dd, 1H), 8.146 (dd, 1H), 7.948–7.916 (m, 1H), 7.798 (q, 1H), 7.716 (m, 2H), 7.520 (q, 1H), 7.448 (dd, 1H), 5.195 (s, 2H, –CH2–CO), and for Bu4N:11 3.209 (m, 4CH2) 1.607 (m, 4CH2) 1.340 (m, 4CH2), 0.970 (t, 4CH3) (see ESI†); ESI-MS: [M − Bu4N+ +H+ + Na+]+, m/z, found, 369.97, calc.: 370.05; 13C NMR (DMSO-d6): 166.346, 149.201, 137.506, 133.612, 131.474, 126.003, 125.667, 125.057, 117.960, 115.223, 46.325.
O 1113. 1H NMR (400 MHz, DMSO-d6): 11.060 (s, 1H), 8.212 (dd, 1H), 8.146 (dd, 1H), 7.948–7.916 (m, 1H), 7.798 (q, 1H), 7.716 (m, 2H), 7.520 (q, 1H), 7.448 (dd, 1H), 5.195 (s, 2H, –CH2–CO), and for Bu4N:11 3.209 (m, 4CH2) 1.607 (m, 4CH2) 1.340 (m, 4CH2), 0.970 (t, 4CH3) (see ESI†); ESI-MS: [M − Bu4N+ +H+ + Na+]+, m/z, found, 369.97, calc.: 370.05; 13C NMR (DMSO-d6): 166.346, 149.201, 137.506, 133.612, 131.474, 126.003, 125.667, 125.057, 117.960, 115.223, 46.325.
X-ray crystallography
X-ray data of the suitable crystal of L was collected on a Bruker's Apex-II CCD diffractometer using Mo Kα (λ = 0.71069). The data were corrected for Lorentz and polarization effects and empirical absorption corrections were applied using SADABS from Bruker. A total of 12449 reflections were measured out of which 3426 were independent and 2801 were observed [I > 2σ(I)] for theta (θ) 1.66 to 28.19°. The structure was solved by direct methods using SIR-92 and refined by full-matrix least squares refinement methods based on F2, using SHELX-97.12 All calculations were performed using Wingx package.13,14 Important crystallographic parameters are given in Table S1.†General method of UV-vis and fluorescence titration
Path length of the cells used for absorption and emission studies was 1 cm. For UV-vis and fluorescence titrations, stock solution of L was prepared in HEPES buffer (1 mM, pH 7.4; 2% EtOH) at 25 °C. Fluorescence measurements were performed using 5 nm × 5 nm slit width. All the fluorescence and absorbance spectra were taken after 30 minutes of mixing of HSO4− and L.Preparation of cell and in vitro cellular imaging with L
Human cervical cancer cell, HeLa cell line was purchased from National Center for Cell Science (NCCS), Pune, India and was used throughout the study. Cell were cultured in Dulbecco's modified Eagle's medium (DMEM, Gibco BRL) supplemented with 10% FBS (Gibco BRL), and 1% antibiotic mixture containing penicillin, streptomycin and neomycin (PSN, Gibco BRL), at 37 °C in a humidified incubator with 5% CO2. For experimental study, cells were grown to 80–90% confluence, harvested with 0.025% trypsin (Gibco BRL) and 0.52 mM EDTA (Gibco BRL) in PBS (phosphate-buffered saline, Sigma Diagnostics) and plated at desire cell concentration and allowed to re-equilibrate for 24 h before any treatment. Cells were rinsed with PBS and incubated with DMEM-containing L (10 μM, 1% DMSO) for 30 min at 37 °C. All experiments were conducted in DMEM containing 10% FBS and 1% PSN antibiotic. The imaging system was composed of a fluorescence microscope (ZEISS Axioskop 2 plus) with an objective lens [10×].Cell cytotoxicity assay
To test the cytotoxicity of L, MTT [3-(4,5-dimethyl-thiazol-2-yl)-2,S-diphenyl tetrazolium bromide] assay was performed with the help of the procedure described earlier.15 After treatments of the probe (5, 10, 20, and 50 μM), 10 μl of MTT solution (10 mg ml−1 PBS) was added in each well of a 96-well culture plate and incubated continuously at 37 °C for 6 h. All mediums were removed from wells and replaced with 100 μl of acidic isopropanol. The intracellular formazan crystals (blue-violet) formed were solubilized with 0.04 N acidic isopropanol and the absorbance of the solution was measured at 595 nm wavelength with a microplate reader. Values are means ± S.D. of three independent experiments. The cell cytotoxicity was calculated as percent cell cytotoxicity = 100% cell viability.Result and discussion
Synthesis and structural characterisation
Organic moiety was synthesized by the reaction of 2-(2-aminophenyl)benzimidazole with 2-chloroacetyl chloride in chloroform solvent followed by ring closer step (Scheme 1) and characterised by physico-chemical and spectroscopic tools (Fig. S1a–d†). The FTIR spectrum of L contains bands for the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and NH groups at 1687 and 3205 cm−1, respectively along with other characteristic peaks for benzimidazole unit (Fig. S1a in ESI†). The QTOF-ESI-MS of L contains molecular ion peak at m/z 249.98 corresponding to [L + H+] (Fig. S1c, ESI†). The well-resolved 1H NMR spectrum of L is in support of the formulation and the structure established by single crystal X-ray crystallographic analysis of HL·NO3.
O and NH groups at 1687 and 3205 cm−1, respectively along with other characteristic peaks for benzimidazole unit (Fig. S1a in ESI†). The QTOF-ESI-MS of L contains molecular ion peak at m/z 249.98 corresponding to [L + H+] (Fig. S1c, ESI†). The well-resolved 1H NMR spectrum of L is in support of the formulation and the structure established by single crystal X-ray crystallographic analysis of HL·NO3.
The solid L–HSO4− ensembled species (2) was obtained from the reaction of tetra butyl ammonium hydrogen sulphate with methanolic solution of L in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mole ratio at stirring condition. The ESI mass spectrum of 2 in methanol shows a molecular-ion peak at m/z 369.97 with ∼14% abundance, which can be assigned to [M − Bu4N+ + H+ + Na+]+ (calculated value at m/z, 370.05). A characteristic peak for νS
1 mole ratio at stirring condition. The ESI mass spectrum of 2 in methanol shows a molecular-ion peak at m/z 369.97 with ∼14% abundance, which can be assigned to [M − Bu4N+ + H+ + Na+]+ (calculated value at m/z, 370.05). A characteristic peak for νS![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O at 1113 cm−1 in the FTIR spectrum of 2 confirms the existence of sulphate ion attached to 2.16 The 1HNMR spectrum obtained in DMSO-d6 confirmed the presence of the L bound to HSO4− ions (Fig. S2a–d†).
O at 1113 cm−1 in the FTIR spectrum of 2 confirms the existence of sulphate ion attached to 2.16 The 1HNMR spectrum obtained in DMSO-d6 confirmed the presence of the L bound to HSO4− ions (Fig. S2a–d†).
Single crystals of the probe (L) were obtained as HL·NO3 from the methanolic solution of L in presence of aq. sodium nitrate. The HL·NO3 crystallizes in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) . An ORTEP view of the probe with atom labelling scheme is illustrated in Fig. 1, and a selection of bond distances and angles is listed in Table S2.† The bond distance C1–N1 (1.3557(18) Å) or C11–N1 (1.4114(17) Å) is somewhat longer than that of any bond of C9–N3 (1.3351(18) Å) or C9–N2 (1.3455(17) Å) due to the single bond character of C1–N1 or C11–N1 and sp3 hybridisation of N1. In the same way, the longer bond distance (1.4602(18) Å) is also indicative of the single bond character of C2–N2 bond. The crystal structure of L is stabilized by the presence of the nitrate anion as counter anion for balancing the produced charge of HL+ formed due to the protonation of N(3) atom.
. An ORTEP view of the probe with atom labelling scheme is illustrated in Fig. 1, and a selection of bond distances and angles is listed in Table S2.† The bond distance C1–N1 (1.3557(18) Å) or C11–N1 (1.4114(17) Å) is somewhat longer than that of any bond of C9–N3 (1.3351(18) Å) or C9–N2 (1.3455(17) Å) due to the single bond character of C1–N1 or C11–N1 and sp3 hybridisation of N1. In the same way, the longer bond distance (1.4602(18) Å) is also indicative of the single bond character of C2–N2 bond. The crystal structure of L is stabilized by the presence of the nitrate anion as counter anion for balancing the produced charge of HL+ formed due to the protonation of N(3) atom.
UV-vis spectroscopic studies of L
UV-vis spectra of L was recorded in HEPES buffer (1 mM, pH 7.4; 2% ethanol) at 25 °C shows an absorption maximum at 383 nm which may possibly be attributed to the intramolecular charge transfer (CT) transition. The absorption intensity of L at 383 nm gradually decreased, accompanied by the formation of a new absorption peak at 425 nm (Fig. 2) as the addition of HSO4− ions was increased stepwise (0–20 μM) with an isosbestic point at 402 nm and the solution turned from colourless to faint greenish yellow (Fig. S3†). | ||
| Fig. 2 UV-vis titration spectra of L (10 μM) upon incremental addition of HSO4− ions (0–20 μM) in HEPES buffer (1 mM, pH 7.4; 2% EtOH) at 25 °C. | ||
Emission study
Organic moiety (L) showed a weak emission at 430 nm excited at 383 nm in HEPES buffer (1 mM, pH 7.4; 2% ethanol) at 25 °C considering the absorption at 383 nm (Fig. S4†). Fluorescence quantum yields (Φ) were estimated by integrating the area under the fluorescence curves with the equation:where A is the area under the fluorescence spectral curve and OD is optical density of the compound at the excitation wavelength, 383 nm, η is the refractive index of the solvent used. The standard used for the measurement of fluorescence quantum yield was anthracene (Φ = 0.29 in ethanol). This emission property of the probe (L) has not been affected over the pH range 4.0–10.0 (Fig. S5†).
Fluorescence studies of L
The emission property of L in presence of various concentrations of HSO4− ions was verified. On addition of various concentrations of HSO4− ions (0–20 μM), fluorescence intensity at 430 nm was significantly decreased with a concomitant increase of intensity at 483 nm through an isoemissive point at 449 nm (Fig. 3). The fluorescence quantum yield has also been calculated in absence and presence of HSO4− ions. And from this measurement it is clear that the fluorescence quantum yield in presence of HSO4− ions (Φ = 0.45) increases ∼4 times than that of free L (Φ = 0.12). This spectral feature for the addition of HSO4− ions was also supported by the fluorescence color change from blue to green in presence of UV light (Fig. S6†). | ||
| Fig. 3 Emission spectra of L in presence of HSO4− ions (0–20 μM) at λex = 383 nm in HEPES buffer (1 mM, pH 7.4; 2% EtOH) at 25 °C. | ||
Ratiometric signaling of fluorescence output at two different wavelengths plotted as a function of concentration of HSO4− ions indicates that the fluorescence intensity ratio of wavelength 483 nm and 430 nm (I483/I430) gradually increases with increase of the concentration of HSO4− ions (Fig. S7†) and after a certain time it level up producing a sigmoid curve. L exhibited a near about 14-fold increase of its fluorescence intensity upon addition of only 2.0 equivalent of HSO4− ions. The detection limit was determined and was found to be 5.5 × 10−7 M−1 (Fig. S8†), which is significantly low. Interestingly, the introduction of other anions does not interfere the fluorescence enhancement. The fluorescence characteristics of L (10 μM) were observed upon the addition of excess 50 equivalents of different anions i.e. F−, Cl−, Br−, I−, CN−, N3– NO3−, ClO4−, H2PO4−, HPO42−, PO43−, H2AsO4−, HAsO42−, AsO33−, OAc−, SO42−, S2−, and HSO4− but only HSO4− ions selectively enhance the fluorescence intensity of L system (Fig. 4). In presence of 10 times excess of various tested anions together with L and HSO4−, almost no adverse effect on intensity was observed (Fig. S9†).
The compound formed between L and HSO4− is found to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in stoichiometry, which was established with the help of Job's plot (Fig. S10†) using the fluorescence study. This 1
1 in stoichiometry, which was established with the help of Job's plot (Fig. S10†) using the fluorescence study. This 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric ratio is also supported by the physico-chemical and spectroscopic data of the L–HSO4− ensemble species isolated in the solid form.
1 stoichiometric ratio is also supported by the physico-chemical and spectroscopic data of the L–HSO4− ensemble species isolated in the solid form.
To ensure the formation of the L–HSO4− species in solution state, 1H NMR titration was also performed in dmso-d6. Fig. 5 clearly indicates the interaction of amide N–H proton with HSO4− ions as the signal due to this proton shifted to downfield (δ value from 10.637 ppm to 11.060 ppm) and the protons of –CH2 of the seven membered ring also shifted to higher δ value from 4.946 ppm to 5.195 ppm along with a significant shifting of Hc towards downfield by Δδ of 0.388 ppm (Δδ = 8.212–7.824 ppm, Fig. S11†) on gradual addition of HSO4− ions.
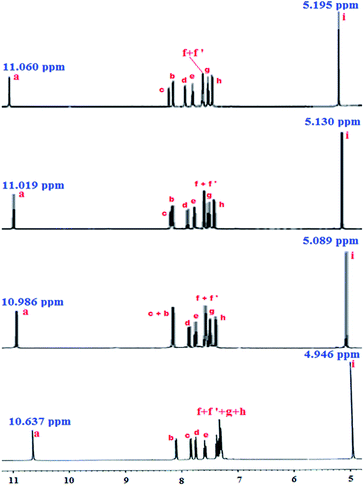 | ||
| Fig. 5 Partial 1H NMR spectra for L (10 mM) in presence of varying [HSO4−] [(A) 0 mM, (B) 3.33 mM, (C) 6.67 mM, and (D) 10 mM] in DMSO-d6 [for clarity, expanded region of 7.2–8.3 ppm of 1HNMR spectra in Fig. S11†]. | ||
The binding constant value was determined from the emission intensity data following the modified Benesi–Hildebrand equation.17,18
| 1/(Fx − F0) = 1/(Fmax − F0) + (1/K[C])(1/(Fmax − F0)) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HSO4−; 1
HSO4−; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5) to 12.15 ns (when L
0.5) to 12.15 ns (when L![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HSO4−; 1
HSO4−; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and it is clearly ascribed by the intermolecular hydrogen bonding assisted CHEF process (Table S3†)(Fig. 6). The strong binding of HSO4− ion with organic moiety (L), is evidenced by the significant binding constant value (4.13 × 106 M−1) and this phenomenon played a key role to deter the PET process in support of the selective detection of HSO4− ions through fluorescence enhancement (Scheme 2).
1), and it is clearly ascribed by the intermolecular hydrogen bonding assisted CHEF process (Table S3†)(Fig. 6). The strong binding of HSO4− ion with organic moiety (L), is evidenced by the significant binding constant value (4.13 × 106 M−1) and this phenomenon played a key role to deter the PET process in support of the selective detection of HSO4− ions through fluorescence enhancement (Scheme 2).
According to the equations:19 τ−1 = kr + knr and kr = Φf/τ, the radiative rate constant kr and total nonradiative rate constant knr of organic moiety, L and HSO4− complex with L were listed in Table S3.† The data suggest that kr has just slightly changed but the factor that induces fluorescent enhancement is mainly ascribed to the decrease of knr.
Geometry optimization
To clarify the configurations and H-bonding feature of the host (L) and guest–host species (2; L–HSO4−), DFT calculations were performed using Gaussian-09 software over a Red Hat Linux IBM cluster. Molecular level interactions between L and 2 have been studied using density functional theory (DFT) with the B3LYP/6-31 G(d) functional model and basis set.To assure the mode of interaction of the probe (L) with HSO4− ions either in ring or chain fashion, the optimised geometries in both fashions were obtained by theoretical calculation (Fig. S13†). From the calculation of energy of HOMO and LUMO in this study, it is indicated that the binding mode of the guest (HSO4− ion) with the host (L) through ring is more preferable than that through chain. In case of ring structure, the energy gap between HOMO-LUMO is 3.77 eV which is lower than that of free L (4.53 eV) (Table S4†). Here, charge distribution of HOMO in compound 2 confirms that the most of the charge is on HSO4− ion attached with N–H through H-bonding (Fig. 7).
Biological studies of L in presence of HSO4−
To examine the utility of the probe in biological systems, it was applied to human cervical cancer HeLa cell. In these experiments both the L and HSO4− ions was allowed to uptake by the cells of interest and the images of the cells were recorded by the fluorescence microscopy following excitation at ∼383 nm. After incubation with L (10 μM) for 30 min, the cells displayed very faint intracellular fluorescence. However, cells exhibited intensive fluorescence when exogenous HSO4− ions was introduced into the cell via incubation with Bu4NHSO4 (Fig. 8). The fluorescence responses of the probe with various concentrations of added HSO4− ions are clearly evident from the cellular imaging. In addition, the in vitro study showed that 10 μM of L did not show no cytotoxic effect to cell upto 6 h (Fig. S14†). These results indicate that the probe has a huge potentiality for both in vitro and in vivo application as HSO4− sensor as well as imaging in different ways as same manner for live cell imaging.Conclusions
In conclusion, a water soluble dual receptor for HSO4− ions was synthesized by a facile two-step process. The formulation and detailed structural characterisations has been established using physico-chemical and spectroscopic tools along with detailed structural analyses by single crystal X-ray crystallography. The fluorimetric and titrimetric titration of L by adding HSO4− ions in HEPES buffer (1 mM, pH 7.4; 2% ethanol) at 25 °C showed that L is very specific and sensitive chemosensor for HSO4− ions. On addition of HSO4− ions, the emission intensity at 430 nm of free L decreases gradually with the generation of a new peak at 483 nm through an isoemissive point at 449 nm due to the hydrogen bonding formation between the amide hydrogen and bisulphate ion. This interaction was also clearly supported by 1HNMR titration and theoretical DFT calculation. Interestingly, this probe could be considered as a potent probe for HSO4− ions because the fluorescence enhancement at λem = 483 nm of bio-friendly visible region is taken place in a more attractive ratiometric response fashion with almost no interference by the biologically relevant anions in aqueous solvent. This probe has been successfully used as biomarker of bisulphate ions as L is significantly efficient to detect the distribution of hydrogen sulphate ions in vitro in living cells (HeLa) in aqueous medium at biological pH by developing the good image.Acknowledgements
Financial assistance from CSIR, New Delhi, India is gratefully acknowledged. B. Sen wishes to thank to UGC, New Delhi, India for offering him the fellowship. The authors sincerely acknowledge Prof. Samita Basu and Mr Ajay Das, Chemical Science Division, SINP, Kolkata for enabling TCSPC instrument, and Prof. B. Mukhopadhyay, IISER, Kolkata for providing the facility to record some 1H NMR spectra and ESI Mass Spectra and also thankful to Mr Samya Banerjee, IISC, Bangalore for recording the 13C NMR spectra.Notes and references
- (a) T. Y. Joo, N. Singh, G. W. Lee and D. O. Jiang, Tetrahedron Lett., 2007, 48, 8846 CrossRef CAS; (b) J. M. Hermida-Ramon and C. M. Estevez, Chem.–Eur. J., 2007, 13, 4743 CrossRef CAS PubMed; (c) Y. Michigami, Y. Kuroda, K. Ueda and Y. Yamamoto, Anal. Chim. Acta, 1993, 274, 299 CrossRef CAS; (d) P. A. Gale, Acc. Chem. Res., 2006, 39, 465 CrossRef CAS PubMed; (e) E. A. Katayev, Y. A. Ustynyuk and J. L. Sesser, Coord. Chem. Rev., 2006, 250, 3004 CrossRef CAS; (f) F. P. Schmidtchen, Coord. Chem. Rev., 2006, 250, 2918 CrossRef CAS; (g) V. Amendola, M. Bonizzoni, D. Esteban-Gomez, L. Fabbrizzi, M. Licchelli, F. Sancenon and A. Taglietti, Coord. Chem. Rev., 2006, 250, 1451 CrossRef CAS; (h) J. M. Llinares, D. Powell and K. Bowman-James, Coord. Chem. Rev., 2003, 240, 57 CrossRef CAS; (i) R. Martınez-Manez and F. Sancenon, Chem. Rev., 2003, 103, 4419 CrossRef PubMed.
- (a) M. E. Moragues, R. Martinez-Manez and F. Sancenon, Chem. Soc. Rev., 2011, 40, 2593 RSC; (b) M. E. Moragues, R. Martınez-Manez and F. Sancenon, Chem. Soc. Rev., 2011, 40, 2593 RSC; (c) A. F. Li, J. H. Wang, F. Wang and Y. B. Jiang, Chem. Soc. Rev., 2010, 39, 3729 RSC; (d) R. M. Duke, E. B. Veale, F. M. Pfeffer, P. E. Kruger and T. Gunnlaugsson, Chem. Soc. Rev., 2010, 39, 3936 RSC; (e) P. A. Gale, Chem. Soc. Rev., 2010, 39, 3746 RSC; (f) E. J. O'Neil and B. D. Smith, Coord. Chem. Rev., 2006, 250, 3068 CrossRef; (g) R. Martinez-Manez and F. Sancenon, Chem. Rev., 2003, 103, 4419 CrossRef CAS PubMed.
- (a) U. Fegade, H. Sharma, K. Tayade, S. Attarde, N. Singh and A. Kuwar, Org. Biomol. Chem., 2013, 11, 6824 RSC; (b) Q. Li, Y. Guo and S. Shao, Analyst, 2012, 137, 4497 RSC; (c) S. T. Yang, D. J. Liao, S. J. Chen, C. H. Hu and A. T. Wu, Analyst, 2012, 137, 1553 RSC; (d) Q. Li, Y. Yue, Y. Guo and S. Shao, Sens. Actuators, B, 2012, 173, 797 CrossRef CAS; (e) K. Kaur, V. K. Bhardwaj, N. Kaur and N. Singh, Inorg. Chem. Commun., 2012, 18, 79 CrossRef CAS; (f) H. J. Kim, S. Bhuniya, R. K. Mahajan, R. Puri, H. G. Liu, K. C. Ko, J. Y. Lee and J. S. Kim, Chem. Commun., 2009, 7128 RSC; (g) R. Shen, X. B. Pan, H. F. Wang, L. H. Yao, J. C. Wu and N. Tang, Dalton Trans., 2008, 3574 RSC.
- (a) J. Chang, Y. Lu, S. He, C. Liu, L. Zhao and X. Zeng, Chem. Commun., 2013, 49, 6259 RSC; (b) W. J. Xue, L. Li, Q. Li and A. X. Wu, Talanta, 2012, 88, 734 CrossRef CAS PubMed; (c) A. Mallick, T. Katayama, Y. Ishibasi, M. Yasudab and H. Miyasaka, Analyst, 2011, 136, 275 RSC.
- (a) Z. Xu, Y. Xiao, X. Qian, J. Cui and D. Cui, Org. Lett., 2005, 7, 889 CrossRef CAS PubMed; (b) S. Sen, T. Mukherjee, S. Sarkar, S. K. Mukhopadhyay and P. Chattopadhyay, Analyst, 2011, 136, 4839 RSC; (c) S. Sen, S. Sarkar, B. Chattopadhyay, A. Moirangthem, A. Basu, K. Dhara and P. Chattopadhyay, Analyst, 2012, 137, 3335 RSC; (d) B. Sen, M. Mukherjee, S. Pal, K. Dhara, S. K. Mandal, A. R. Khuda-Bukhsh and P. Chattopadhyay, RSC Adv., 2014 10.1039/C3RA47800A.
- B. Valeur and I. Leray, Coord. Chem. Rev., 2000, 205, 3 CrossRef CAS.
- (a) Z. Yang, K. Zhang, F. Gong, S. Li, J. Chen, J. S. Ma, L. N. Sobenina, A. I. Mikhaleva, G. Yang and B. A. Trofimov, Beilstein J. Org. Chem., 2011, 7, 46 CrossRef CAS PubMed; (b) T. Gunnlaugsson, M. Glynn, G. M. Tocci, P. E. Kruger and F. M. Pfeffer, Coord. Chem. Rev., 2006, 250, 3094 CrossRef CAS; (c) R. Martinez-Manez and F. Sancanon, Chem. Rev., 2003, 103, 4419 CrossRef CAS PubMed; (d) J. F. Callan, A. P. de Silva and D. C. Magri, Tetrahedron, 2005, 61, 8551 CrossRef CAS; (e) J. Zhao, T. M. Fyles and T. D. James, Angew. Chem., Int. Ed., 2004, 43, 3461 CrossRef CAS PubMed; (f) C. H. Lee, H. Miyaji, D. W. Yoon and J. L. Sessler, Chem. Commun., 2008, 24 RSC; (g) S. K. Kim, D. H. Lee, J. I. Hong and J. Yoon, Acc. Chem. Res., 2009, 42, 23 CrossRef CAS PubMed; (h) X. Huang, Z. Guo, W. Zhu, Y. Xie and H. Tian, Chem. Commun., 2008, 5143 RSC.
- P. A. Gale, in The Encyclopedia of Supramolecular Chemistry, ed. J. L. Atwood and J. W. Steed, Dekker, New York, 2004, pp. 31–41 Search PubMed.
- R. A. Pascal Jr, J. Spergel and D. Van Engen, Tetrahedron Lett., 1986, 27, 4099 CrossRef.
- X. Zhou, P. Li, Z. Shi, X. Tang, C. Chen and W. Liu, Inorg. Chem., 2012, 51, 9226 CrossRef CAS PubMed.
- B. Das, S. Sarkar, A. Patra, M. G. B. Drew and P. Chattopadhyay, J. Coord. Chem., 2008, 61, 1689 CrossRef CAS.
- A. Altomare, G. Cascarano, C. Giacovazzo and A. Guagliardi, J. Appl. Crystallogr., 1993, 26, 343 CrossRef.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112 CrossRef CAS PubMed.
- L. J. Farrugia, J. Appl. Crystallogr., 1999, 32, 837 CrossRef CAS.
- J. Ratha, K. A. Majumdar, S. K. Mandal, R. Bera, C. Sarkar, B. Saha, C. Mandal, K. D. Saha and R. Bhadra, Mol. Cell. Biochem., 2006, 290, 113 CrossRef CAS PubMed.
- J. Zhou, D. T. Moore, L. Wöste, G. Meijer, D. M. Neumark and K. R. Asmis, J. Chem. Phys., 2006, 125, 111102 CrossRef PubMed.
- A. Mallick, B. Haldar and N. Chattopadhyay, Photochem. Photobiol., 2005, 78, 215 CrossRef CAS PubMed.
- H. A. Benesi and J. H. Hildebrand, J. Am. Chem. Soc., 1949, 71, 2703 CrossRef CAS.
- N. J. Turro, Modern Molecular Photochemistry; Benjamin/Cummings Publishing Co., Inc., Menlo Park, CA, 1978 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 972698. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ra00291a |
| This journal is © The Royal Society of Chemistry 2014 |








