A non-platonic M4L4 complex constructed using heterotopic ligands†
Gregory S. Hall,
Adrian J. Emerson and
David R. Turner*
School of Chemistry, Monash University, Clayton, Vic 3800, Australia. E-mail: david.turner@monash.edu; Fax: +61 (0)3 9905 4597; Tel: +61 (0)3 9905 6293
First published on 7th February 2014
Abstract
An unusual, chloride-capped M4L4 complex has been prepared using heterotopic ligands with carboxylate and dipyridyl coordinating sites. The incorporation of low symmetry, heterotopic ligands into the M4L4 architecture gives rise to a non-platonic complex with irregular triangular faces, demonstrating that the standard high-symmetry model traditionally adopted for synthesising such complexes is not a strict rule. The M4L4 compound is also observed by mass spectrometry in solution, yet shows no indication of host–guest behaviour in its small cavity. The assembly of the complex is assisted by a ‘belt’ of CH⋯π interactions.
Introduction
Maintaining control over self-assembly processes, whilst almost an oxymoronic sentiment, is an important goal in supramolecular chemistry.1 Judicious selection of ligands and metal ions with known predispositions towards certain coordination geometries allows topological arguments to be made in favour of forming desired structures. However, changing conditions such as temperature, pressure, concentrations, solvents or metal coordination geometry are observed to have a marked effect on the products that are obtained.Some of the key targets that are derived from self-assembling systems are discrete metal–organic cages which are usually designed to be highly symmetric.2 This type of species is of interest for their potential to safely store reactive molecules,3 to selectively sense small molecules/ions4 or to catalyse/control reactions in environments that are separated from the bulk media.5 There have been many elegant reports of tetrahedral cages being constructed as either M4L6 systems, in which the ligands form the edges of the cage,6 or M4L4 cages in which the ligands form the triangular faces of the tetrahedron.7 In the latter case the design of ligands is typically focussed on molecules that possess C3 symmetry, for example, species based around 1,3,5-substituted benzene, tertiary amines or boron-centred molecules.8 Designed syntheses typically rely on symmetry-driven routes towards polyhedral assemblies whether by ‘panelling’ the faces of such species or using linear ditopic species to act as the edges.9
The use of low-symmetry ligands to form metal–organic polyhedra is a less commonly used synthetic strategy due to the inherently larger library of potential products including those from linkage isomerism.1c,10 Examples exist of polygons11 and metallomacrocycles12 that have been synthesised using this approach in addition to a range of coordination polymers assembled with ambidentate pyridyl/carboxylate ligands.13
Herein we describe an unusual M4L4 complex which demonstrates that distorted polyhedra can be constructed without high internal symmetry by using low symmetry pyridyl/carboxylate ligands.
Experimental details
Synthetic details
All reagents except solvents were purchased from standard commercial suppliers and were used without further purification. Acetonitrile and methanol were dried and stored over 3 Å molecular sieves. NMR spectra were recorded using a Bruker Avance400 spectrometer operating at 400 and 100 MHz for 1H and 13C, respectively. Mass spectra of organic compounds were recorded using a Micromass platform 2 spectrometer with an electrospray source and a cone voltage of 35 V. Infrared spectra were recorded using a Bruker Equinox 55 diamond anvil Attenuated Total Reflection (ATR) spectrometer using Opus-6 software system.X-ray crystallography
Data for [Cu4Cl2(L2)4]·2ClO4 (1) and [Cu(L2H)2]·2ClO4·2H2O (2) were collected using the MX1 beamline at the Australian Synchrotron, Victoria, Australia. The wavelength was set at 0.7107 Å (17.4 keV) and data collection temperatures were maintained at 100 K using an open-flow N2 cryostream. Data collection was conducted using the BluIce package.14 Indexing and integration was conducted using the program XDS.15 Structures were solved by direct methods using SHELXS-97 (ref. 16) and were refined by alternating least-squares cycles using SHELXL-97 (ref. 16) with X-Seed as a graphical interface.17 All non-hydrogen atoms were refined using an anisotropic model. Hydrogen atoms attached to carbon were placed in idealised positions and refined using a riding model in both structures. Additional refinement details specific to each structure are given in the ESI.†![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3280 reflections collected, 40
3280 reflections collected, 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 546 unique (Rint = 0.0532). Final GooF = 1.032, R1 = 0.0751, wR2 = 0.2061, R indices based on 33
546 unique (Rint = 0.0532). Final GooF = 1.032, R1 = 0.0751, wR2 = 0.2061, R indices based on 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 419 reflections with I > 2sigma(I) (refinement on F2), 2314 parameters, 113 restraints, μ = 1.157 mm−1.
419 reflections with I > 2sigma(I) (refinement on F2), 2314 parameters, 113 restraints, μ = 1.157 mm−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 145 reflections collected, 6297 unique (Rint = 0.1035). Final GooF = 1.058, R1 = 0.0624, wR2 = 0.1828, R indices based on 4954 reflections with I > 2sigma(I) (refinement on F2), 317 parameters, 4 restraints, μ = 0.757 mm−1.
145 reflections collected, 6297 unique (Rint = 0.1035). Final GooF = 1.058, R1 = 0.0624, wR2 = 0.1828, R indices based on 4954 reflections with I > 2sigma(I) (refinement on F2), 317 parameters, 4 restraints, μ = 0.757 mm−1.Results and discussion
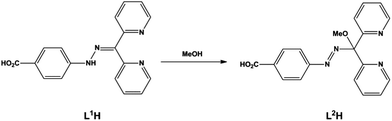
The ambidentate species L1H (Scheme 1) was prepared as a hemihydrochloride salt in a quantitative yield by the reaction of 4-hydrazinobenzoic acid with 2,2′-dipyridylketone. The ligand is of low symmetry and is heterotopic, having different coordinating groups at the two ends. Furthermore, both the carboxylate and the dipyridyl group are capable of either chelating or bridging, potentially giving the ligand a significant degree of freedom in its coordination behaviour.Reactions of L1H·1/2HCl with either [Cu(MeCN)4](ClO4) or Cu(ClO4)2 in hot methanol each yield a yellow crystalline material after vapour diffusion of 2,6-lutidine in to the reaction mixture. These products were analysed by both single crystal and powder X-ray diffraction and were found to contain a novel chloride-capped M4L4 complex [Cu4Cl2(L2)4]·2ClO4 (1) with the formation of the mononuclear by-product [Cu(L2H)2]·2ClO4·2H2O (2) and minor impurity of [Cu(2,6-lutidine)2Cl2]. Whilst the Cu(I) and Cu(II) starting materials both gave the same products the overall yield was notably better using the divalent precursor. Direct addition of base to the reaction mixtures result only in the rapid precipitation of an amorphous material. Despite repeated attempts under a variety of conditions, the compounds could not be isolated independently of each other. Compounds 1 and 2 contain the L2 or L2H ligand, respectively, which result from the in situ addition of methanol to L1H (Scheme 1) as is well documented for dipyridylketone derivatives.18 The L2 species has the ability to coordinate in various manners owing to the potentially divergent carboxylate group at one end and free rotation of the two pyridyl groups at the other end which overall gives the prospect of a tetra-bridging ligand.
The unusual M4L4 complex that is formed, [Cu4Cl2(L2)4]·2ClO4 (1), crystallises in P21/c with disordered counter-anions and some solvent-filled void space (see ESI†). The asymmetric unit of the structure contains two unique [Cu4Cl2(L2)4]2+ complexes and associated anions. The dicationic complex contains two Cu2 units in which the metal atoms are bridged by a μ2 chloride (from the hydrochloride starting material) and two carboxylate groups from L2 ligands (Fig. 1). The four L2 ligands are arranged in an alternating head-to-tail manner with the Cu⋯Cu vectors of the Cu2 units at the ‘top’ and ‘bottom’ of the complex perpendicular to each other. The dipyridyl units chelate to two of the remaining coordination sites of the Cu atoms making each 5-coordinate with square-pyramidal geometries and O2N2Cl coordination spheres. The bridging chloride ligand occupies the elongated apical position of the copper coordination sphere with the Cu atoms raised an average of 0.27 Å out of the mean O2N2 plane in the direction of the chloride. Each Cu atom has apparent long interactions to one nitrogen atom from a hydrazone group in the range 2.59–2.62 Å thereby not fully completing the N3 tridentate coordination from the ligand which is observed in the mononuclear complex [Cu(L2H)2]2+ (vide infra). These nitrogen atoms are observed to coordinate in a way which is related to the bis(dipyridylketone) ligands reported by Wu et al.18
 | ||
| Fig. 1 A schematic representation of the non-platonic M4L4 complex [Cu4Cl2(L2)4]2+ (only one ligand shown for clarity). | ||
Each of the L2 ligands can be thought of as acting as the triangular face of a highly distorted, non-platonic tetrahedron with each oxygen atom acting as one corner and the chelating dipyridyl group as the third. The ‘tetrahedron’ is distorted in regard to the positions of the Cu atoms with distances of approximately 3.5 Å between the carboxylate-bridged copper atoms and 9 Å between metal atoms across the long edges of the complex. However, if vectors are drawn between the sp3 carbon atoms of the L2 ligands they describe an near-regular tetrahedron with C⋯C separations in the range 9.52–9.67 Å across the Cu2 units and 10.94–11.28 Å along the longer edges (Fig. 2). This arrangement is distinct to the M4L4 complexes that are reported using symmetric ligands in which a true tetrahedral cage is formed with the metal ions residing at the vertices and the complex in 1 is therefore not classed as a tetrahedral complex. The formation of the complex appears to be assisted by edge-to-face CH⋯π interactions between the central aromatic rings of the L2 ligands (Fig. 3). These interactions form a continuous ‘belt’ around the centre of the complex with H⋯centroid distances in the range 2.46–2.69 Å. Whilst it appears that this hydrogen-bonding arrangement may have some influence on the assembly of the complex the ‘belt’ also restricts the size of the internal cavity of the M4L4 complex. No evidence is observed of any guest inclusion within the complex which is slightly too small to accommodate any solvent molecules.
 | ||
| Fig. 2 The structure of the complex [Cu4Cl2(L2)4]2+ as determined from X-ray diffraction data showing the distorted tetrahedral shape described by the sp3 carbon atoms of the L2 ligands. | ||
 | ||
| Fig. 3 Space-filling representation of [Cu4Cl2(L2)4]2+ showing the CH⋯π interactions that form a ‘belt’ around the middle of the complex. | ||
In the crystal structure of 1, the complexes pack in one-dimensional chains with weak π–π interactions between adjacent complexes with neighbouring complexes approximately perpendicular to each other. Space between the chains is occupied by disordered counter-anions and volatile species. Solution speciation is difficult to determine as NMR spectra of the paramagnetic Cu(II) complexes contain extremely broad signals. Mass spectrometry clearly shows the presence of both the M4L4 complex 1 and the mononuclear species 2 (see ESI†). There is no evidence from the mass spectrometry results that complex 1 is able to incorporate small solvent molecules (water, methanol or acetonitrile) as was expected from the structure of the complex as determined by X-ray diffraction.
The second product that is obtained from the reaction of L1H with Cu(I) or Cu(II) perchlorate is the mononuclear complex [Cu(L2H)2]2+ which is obtained as the solvated structure [Cu(L2H)2](ClO4)2·2H2O. Two L2H ligands coordinate to the copper atom, which lies on a crystallographic inversion centre in the P21/c structure, in a tridentate manner through the nitrogen atoms of the two pyridyl groups and one of the nitrogen atoms from the hydrazone group (Fig. 4a). The nitrogen atoms from the hydrazone groups occupy the axial sites in the Jahn–Teller distorted coordination sphere with a Cu–N distance of 2.437(2) Å which is much shorter than the long ‘contact’ arising from the same nitrogen atom in the M4L4 complex (vide supra).
In the [Cu(L2H)2]2+ complex the L2H ligand remains protonated at the carboxylic acid and as such takes part in hydrogen-bonding interactions rather than coordinating to a metal atom. The dicationic complexes arrange in to one-dimensional chains by hydrogen-bonding rings that incorporate the water molecules to give an R44(12) motif (Fig. 4b). The remaining hydrogen atom of the water molecule forms an interaction with the perchlorate counter-anion.
Conclusions
The chloride-capped, M4L4 complex [Cu4Cl2(L2)4]2+ has been synthesised from a self-assembly process involving low-symmetry heterotopic ligands. This cage self-assembles against the generally held tenet that high symmetry ligand species are necessary to form polyhedral complexes. Current work seeks to expand upon these results to synthesise larger systems.Acknowledgements
DRT acknowledges the Australian Institute of Nuclear Science and Engineering (AINSE) and the Australian Research Council (ARC) for funding and fellowships. GSH acknowledges AINSE for a PRGA top-up scholarship. Part of this work was undertaken using the MX1 beamline at the Australian Synchrotron, Victoria, Australia. We thank Dr Marie Squire, University of Canterbury, New Zealand, for mass spectrometry of metal complexes.Notes and references
- (a) J. W. Steed and J. L. Atwood, Supramolecular chemistry, John Wiley & Sons, Chichester, 2009 Search PubMed; (b) J. W. Steed, D. R. Turner and K. J. Wallace, Core concepts in supramolecular chemistry and nanochemistry, John Wiley, Chichester, England, Hoboken, NJ, 2007 Search PubMed; (c) B. H. Northrop, Y. R. Zheng, K. W. Chi and P. J. Stang, Acc. Chem. Res., 2009, 42, 1554–1563 CrossRef CAS PubMed.
- (a) D. R. Turner, A. Pastor, M. Alajarin and J. W. Steed, Struct. Bonding, 2004, 108, 97–168 CrossRef CAS; (b) S. R. Seidel and P. J. Stang, Acc. Chem. Res., 2002, 35, 972–983 CrossRef CAS PubMed.
- (a) P. Mal, B. Breiner, K. Rissanen and J. R. Nitschke, Science, 2009, 324, 1697–1699 CrossRef CAS PubMed; (b) I. A. Riddell, M. M. J. Smulders, J. K. Clegg and J. R. Nitschke, Chem. Commun., 2011, 47, 457–459 RSC.
- (a) J. K. Clegg, J. Cremers, A. J. Hogben, B. Breiner, M. M. J. Smulders, J. D. Thoburn and J. R. Nitschke, Chem. Sci., 2013, 4, 68–76 RSC; (b) S. Zarra, M. M. J. Smulders, Q. Lefebvre, J. K. Clegg and J. R. Nitschke, Angew. Chem., Int. Ed., 2012, 51, 6882–6885 CrossRef CAS PubMed; (c) D. Fiedler, D. H. Leung, R. G. Bergman and K. N. Raymond, Acc. Chem. Res., 2005, 38, 349–358 CrossRef CAS PubMed; (d) C. R. K. Glasson, J. K. Clegg, J. C. McMurtrie, G. V. Meehan, L. F. Lindoy, C. A. Motti, B. Moubaraki, K. S. Murray and J. D. Cashion, Chem. Sci., 2011, 2, 540–543 RSC.
- (a) M. M. J. Smulders and J. R. Nitschke, Chem. Sci., 2012, 3, 785–788 RSC; (b) B. Breiner, J. K. Clegg and J. R. Nitschke, Chem. Sci., 2011, 2, 51–56 RSC; (c) M. Yoshizawa, J. K. Klosterman and M. Fujita, Angew. Chem., Int. Ed., 2009, 48, 3418–3438 CrossRef CAS PubMed; (d) M. D. Pluth, R. G. Bergman and K. N. Raymond, Science, 2007, 316, 85–88 CrossRef CAS PubMed; (e) W. M. Hart-Cooper, K. N. Clary, F. D. Toste, R. G. Bergman and K. N. Raymond, J. Am. Chem. Soc., 2012, 134, 17873–17876 CrossRef CAS PubMed.
- K.-C. Sham, G. Zheng, Y. Pan, K.-C. Lau, S.-M. Yiu and H.-L. Kwong, Inorg. Chem. Commun., 2012, 24, 70–72 CrossRef CAS PubMed.
- (a) R. M. Yeh, J. Xu, G. Seeber and K. N. Raymond, Inorg. Chem., 2005, 44, 6228–6239 CrossRef CAS PubMed; (b) M. Albrecht, Y. Shang, T. Rhyssen, J. Stubenrauch, H. D. F. Winkler and C. A. Schalley, Eur. J. Org. Chem., 2012, 2422–2427 CrossRef CAS.
- (a) M. Albrecht and R. Froehlich, Bull. Chem. Soc. Jpn., 2007, 80, 797–808 CrossRef CAS; (b) P. J. Steel, Acc. Chem. Res., 2005, 38, 243–250 CrossRef CAS PubMed.
- D. L. Caulder and K. N. Raymond, J. Chem. Soc., Dalton Trans., 1999, 1185–1200 RSC.
- L. Zhao, B. H. Northrop, Y.-R. Zheng, H.-B. Yang, H. J. Lee, Y. M. Lee, J. Y. Park, K.-W. Chi and P. J. Stang, J. Org. Chem., 2008, 73, 6580–6586 CrossRef CAS PubMed.
- (a) S. Ghosh, D. R. Turner, S. R. Batten and P. S. Mukherjee, Dalton Trans., 2007, 1869–1871 RSC; (b) S. Ghosh and P. S. Mukherjee, Inorg. Chem., 2009, 48, 2605–2613 CrossRef CAS PubMed; (c) P. Teo, L. L. Koh and T. S. A. Hor, Inorg. Chem., 2008, 47, 6464–6474 CrossRef CAS PubMed; (d) P. Teo, L. L. Koh and T. S. A. Hor, Dalton Trans., 2009, 5637–5646 RSC.
- (a) K. W. Chi, C. Addicott, A. M. Arif and P. J. Stang, J. Am. Chem. Soc., 2004, 126, 16569–16574 CrossRef CAS PubMed; (b) A. K. Bar, R. Chakrabarty, H. M. Lee and P. S. Mukherjee, Inorg. Chim. Acta, 2011, 372, 313–320 CrossRef CAS PubMed; (c) K. W. Chi, C. Addicott, M. E. Moon, H. J. Lee, S. C. Yoon and P. J. Stang, J. Org. Chem., 2006, 71, 6662–6665 CrossRef CAS PubMed.
- P. Teo and T. S. A. Hor, Coord. Chem. Rev., 2011, 255, 273–289 CrossRef CAS PubMed.
- T. M. McPhillips, S. E. McPhillips, H. J. Chiu, A. E. Cohen, A. M. Deacon, P. J. Ellis, E. Garman, A. Gonzalez, N. K. Sauter, R. P. Phizackerley, S. M. Soltis and P. Kuhn, J. Synchotron Rad., 2002, 9, 401–406 CAS.
- W. Kabsch, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2010, 66, 125–132 CrossRef CAS PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. A, 2008, 64, 112–122 CrossRef CAS PubMed.
- L. J. Barbour, J. Supramol. Chem., 2001, 1, 189–191 CrossRef CAS.
- D.-Y. Wu, W. Huang, W.-J. Hua, Y. Song, C.-Y. Duan, S.-H. Li and Q.-J. Meng, Dalton Trans., 2007, 1838–1845 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: CIFs for X-ray structures of 1 and 2, additional crystallographic information, mass spectrometry and PXRD details. CCDC 925296 and 925297. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c4ra00241e |
| This journal is © The Royal Society of Chemistry 2014 |