Lithium iron(II) pyrophosphate as a cathode material: structure and transport studies†
Prashant Dabas* and
K. Hariharan
Department of Physics, Indian Institute of Technology Madras, Chennai, India. E-mail: prashant@physics.iitm.ac.in; haran@iitm.ac.in; Fax: +91 044 22574852; Tel: +91 044 22574856
First published on 31st January 2014
Abstract
A single phase cathode material Li2FeP2O7 was synthesized by a solid state reaction using inexpensive precursors and optimization of the calcination conditions. Reitveld refinement of the XRD data reveals the value of Rwp and reduced-χ2 as 0.97% and 1.006, respectively. Impedance spectroscopy indicates an electronically dominated conduction mechanism with a conductivity of 3.4 × 10−9 S cm−1 at 303 K.
Lithium ion secondary batteries are one of the most promising alternate energy sources that provide a high energy density and operating voltage which are useful for a range of applications from household devices to hybrid electric vehicles (HEV).1–3 The cathode materials in lithium ion secondary batteries not only dictate its capacity and cycle life, but also determine the cost and safety. Lithium cobalt oxide (LiCoO2) showing an average potential of 3.9 V with a practical capacity of 150 mA h g−1 is a successfully commercialized cathode material.4 However, the high cost and adverse effects of cobalt on the environment have directed researchers to search for cathode materials based on other transition metals. Olivine structured materials, such as lithium iron phosphate (LiFePO4), are anticipated to be promising cathode materials as they portray appreciable electrochemical properties (average potential 3.4 V, practical capacity 160 mA h g−1).4 However, the poor electronic conductivity of LiFePO4 hinders its commercial usage. In order to improve the conduction characteristics several methods, such as decreasing the particle size, doping ions such as Zr4+, Ti4+, V5+ at Fe sites, coating LiFePO4 particles with carbonaceous compounds, fluorination of LiFePO4, have been attempted.5,6
On the other hand, the search has already begun for novel cathode materials. Recently, a few pyrophosphate based Li2MP2O7 (M = Mn, Fe, Co) materials have been synthesized and tested for application as cathode materials.7–13 Out of these only Li2FeP2O7 showed appreciable electrochemical activity with an average voltage of 3.5 V and practical capacity of 85 mA h g−1. Li2MnP2O7 was found to be electrochemically inactive while Li2CoP2O7 depicted a voltage of 4.9 V which is higher than the breakdown voltage of most electrolytes. Attempts have also been made to refine the structure of Li2FeP2O7.8–10 These reports mention the presence of impurity phases in Li2FeP2O7.
We present here the exact preparation conditions to obtain pure phase Li2FeP2O7 and the precise crystallographic data. Electrochemical data concerning Li2FeP2O7 has been reported in the literature but no report seems to be available on the conduction characteristics. We have investigated the basic electrical properties of Li2FeP2O7 using impedance spectroscopy as an experimental tool.
Fig. 1 shows a schematic for the preparation of Li2FeP2O7. In order to find the exact phase formation temperature and the reason behind the presence of the impurity phases, such as Fe2P2O7, LiFePO4 and LiFeP2O7 in Li2FeP2O7,8,10 the XRD patterns were recorded after each sintering temperature up to 873 K and it is observed that the impurity phase LiFePO4 creeps-in upon calcining at temperatures above 853 K. In previous reports the samples were calcined at higher temperatures leading to the formation of impurity phases. Also, we prepared the cathode material from inexpensive and easily available starting materials.
A probable reason for the impurity phase (LiFePO4) may be the decomposition of P2O74− units (pyrophosphate) to PO43− (orthophosphate) and PO3− units (metaphosphate) due to prolonged heat treatment at higher temperatures.14 The impurity LiFeP2O7 is avoided by calcining the sample in a reductive atmosphere of 85% Ar + 15% H2. The impurity phase Fe2P2O7 may be present due to some initial non-stiochiometry, however, no such phase is observed in our sample (Fig. 2). Room temperature Rietveld refinement of the X-ray diffraction data for the Li2FeP2O7 sample is shown in Fig. 2. The data was refined using the General Structure Analysis System (GSAS) software.15 Shifted Chebyschev function was used to refine the background while the pseudo-Voigt function was used to refine the profile shape. The Bragg peaks are indexed using the monoclinic space group P21/c (no. 14). The refined lattice parameters are a = 11.02260(9) Å, b = 9.75443(10) Å, c = 9.80898(7) Å, β = 101.5321(8)° and V = 1033.363(16) Å3. The fitting parameters (Rwp = 0.97%, Rp = 0.75%, goodness of fit (GOF), reduced-χ2 = 1.006) obtained are better than those mentioned in previous reports which establish the phase purity of the above prepared Li2FeP2O7. The fractional atomic coordinates, occupancies and the thermal displacement parameters are listed in the ESI.†
Fig. 3 shows the refined crystal structure of Li2FeP2O7 drawn using Vesta software.16 The structure of Li2FeP2O7 reveals that the Fe1 sites are octahedrally coordinated as FeO6 while the Fe2 and Fe3 sites are coordinated as distorted FeO5 trigonal bipyramids. The P atoms are coordinated as corner sharing PO4 tetrahedra forming the P2O74− units. The Fe polyhedron are edge sharing and the three edge sharing Fe polyhedron share a corner with one of the PO4 tetrahedron of the P2O74− unit. The Fe2 and Fe3 atoms share their sites with lithium atoms Li5 and Li4, respectively. The Li1 and Li3 atoms exist as edge sharing distorted LiO5 trigonal bipyramids, while Li2 exists as a LiO4 tetrahedron. The Li1, Li2 and Li3 atoms are stacked along the b–c plane, which may provide the possible pathways for lithium ion diffusion (Fig. 3).
A standard redox titration of Mohr's salt and potassium dichromate was used to estimate the total iron content (Fe2+ and Fe3+) and Fe2+ in Li2FeP2O7.17 A 0.05 N solution of Mohr's salt containing 50 mg of Li2FeP2O7 is titrated against 0.05 N solution of K2Cr2O7 revealing that the total iron content and Fe2+ content in the sample are 11.43 and 11.40 mg, respectively. The above quantitative analysis establishes that iron is present only as Fe2+ in the above prepared Li2FeP2O7.
The stoichiometry of the sample was also checked by Energy-Dispersive X-ray Spectroscopy (EDS) studies (Fig. 4). The average crystallite size as calculated using the Scherrer formula, D = 0.9λ/(β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ) (D = crystallite size, λ = wavelength of X-ray, β = Full Width at Half Maxima (FWHM)) turns out to be 0.3 μm. The FWHM used for the calculation was obtained from the Rietveld refinement and was hence free from instrumental and temperature factors. The SEM image gives the average particle size as ∼1 μm (inset of Fig. 4) indicating each particle consists of several crystallites.
θ) (D = crystallite size, λ = wavelength of X-ray, β = Full Width at Half Maxima (FWHM)) turns out to be 0.3 μm. The FWHM used for the calculation was obtained from the Rietveld refinement and was hence free from instrumental and temperature factors. The SEM image gives the average particle size as ∼1 μm (inset of Fig. 4) indicating each particle consists of several crystallites.
 | ||
| Fig. 4 Energy-dispersive X-ray spectroscopy (EDS) for lithium iron(II) pyrophosphate. Inset shows an SEM image of the sample indicating the particle size. | ||
Fig. 5 shows the TGA and DSC scans for Li2FeP2O7. The DSC scan does not show any transition up to the melting peak, which is observed at 988 K. Also, no appreciable weight loss is observed in the sample up to the melting temperature. The above observations indicate that the cathode material is thermally stable up to its melting point. The thermal stability may be attributed to the presence of corner sharing PO4 tetrahedrons forming the P2O74− units in the structure (Fig. 3).
 | ||
| Fig. 5 Thermogravimetric Analysis (TGA) and Differential Scanning Calorimetry (DSC) of lithium iron(II) pyrophosphate at a heating rate of 10 K min−1. | ||
To carry out the conductivity measurements, the sample was pelletized and sintered progressively up to a temperature of 853 K. The density of the pellet was calculated using the Archimedes principle to be 3.02 g cm−3 (96.4% of the theoretical value). Silver electrodes were applied on the opposite faces of the pellet and it was loaded in a spring tight arrangement. Potentiostatic DC polarization measurements were performed to quantify the electronic transport. A constant voltage was applied to the ion blocking cell assembly and the resulting current was recorded as a function of time.
Fig. 6 shows the variation of polarization current as a function of time at a typical temperature of 503 K. A high temperature was chosen for the measurement to observe the signature of any ionic transport. The current increased in a step towards a stationary value as soon as the voltage was applied and decreased in a corresponding manner on turning off the DC voltage, indicating the conductivity is dominated by the electrons in Li2FeP2O7.
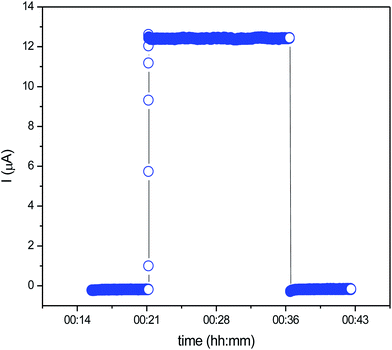 | ||
| Fig. 6 Variation of polarization current as a function of time at 503 K, the DC voltage applied was 0.2 V. | ||
Furthermore, Fig. 7 shows the complex impedance plot at four typical temperatures. Depressed semicircles are observed in the temperature range 293–473 K indicating a distribution of relaxation times. However, the presence of a single depressed semicircle circle is consistent with the absence of any other impurity phase and grain boundaries in the sample.
 | ||
| Fig. 7 Complex impedance plot for the cathode material lithium iron(II) pyrophosphate at four typical temperatures. | ||
The impedance data can also be analyzed in conductivity formalism (σ′ = (Z′/|Z|2)(L/A), where L is the thickness and A is the area of cross-section of the pellet) and several important parameters, such as hopping frequency and activation for the conduction process, can be calculated. Fig. 8 shows the variation of the real part of conductivity (σ′(ω)) with frequency (ω) at four typical temperatures. The data can be analyzed using the Almond–West relation18
| σ′(ω) = σdc[1 + (ω/ωc)s] | (1) |
 | ||
| Fig. 8 AC conductivity as a function of frequency. Solid lines represent the fitting using the Almond–West relation. The inset shows the variation of dc conductivity (σdc) with temperature. | ||
| Temperature (K) | σdc (S cm−1) | s | ωc (rad s−1) |
|---|---|---|---|
| 293 | 1.8 ± 0.1 × 10−9 | 0.65 | 1.9 × 103 |
| 303 | 3.4 ± 0.1 × 10−9 | 0.65 | 3.6 × 103 |
| 313 | 6.2 ± 0.1 × 10−9 | 0.66 | 6.9 × 103 |
| 323 | 1.1 ± 0.1 × 10−8 | 0.66 | 1.2 × 104 |
Conclusions
In summary, we have synthesized pure phase Li2FeP2O7 by a solid state reaction method using cheap starting materials. Impurity phases, such as LiFePO4 and LiFeP2O7, are avoided by calcining the sample only up to 853 K in a reductive environment. Rietveld refinement of the sample reveals that Li2FeP2O7 crystallizes in the monoclinic space group P21/c with lattice parameters a = 11.02260(9) Å, b = 9.75443(10) Å, c = 9.80898(7) Å and β = 101.5321(8)°. The fitting parameters (Rwp = 0.97%, GOF = 1.006) are better than those obtained in previous reports. The structural studies reveal that the lithium ions are stacked around the b–c plane which may provide possible diffusion pathways for lithium ions. The structure consists of corner sharing PO4 tetrahedrons which provide thermal stability to the structure, as evident from the TGA and DSC studies. DC polarization and impedance spectroscopy measurements indicate an electronically dominated conduction mechanism with a conductivity of 3.4 × 10−9 S cm−1 at 303 K. The conduction process was found to be thermally activated with an activation energy of 0.52 eV.Notes and references
- M. Armand and J. M. Tarascon, Nature, 2008, 451, 652 CrossRef CAS PubMed.
- J. W. Fergues, J. Power Sources, 2010, 195, 939 CrossRef.
- L. X. Yuan, Z. H. Wang, W. X. Zhang, X. L. Hu, J. T. Chen, Y. H. Huang and G. B. Goodenough, Energy Environ. Sci., 2012, 5, 5163 Search PubMed.
- J. K. Park, Principles and Applications of Lithium Secondary Batteries, Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 2012, pp. 21–87 Search PubMed.
- W. J. Zhang, J. Power Sources, 2011, 196, 2962 CrossRef CAS.
- M. Ueda, M. Ohe, J. H. Kim, S. Yonezawa and M. Takashima, J. Fluorine Chem., 2013, 149, 88 CrossRef CAS.
- L. Adam, A. Guesdon and B. Raveau, J. Solid State Chem., 2008, 181, 3110 CrossRef CAS.
- S. Nishimura, M. Nakamura, R. Natsui and A. Yamada, J. Am. Chem. Soc., 2010, 132, 13596 CrossRef CAS PubMed.
- H. Zhou, S. Upetri, N. A. Chernova, G. Hautier, G. Ceder and M. S. Whittingham, Chem. Mater., 2011, 23, 293 CrossRef CAS.
- H. Kim, S. Lee, Y. U. Park, H. Kim, J. Kim, S. Jeon and K. Kang, Chem. Mater., 2011, 23, 3930 CrossRef CAS.
- M. Tamaru, S. C. Chung, D. Shimizu, S. Nishimura and A. Yamada, Chem. Mater., 2013, 25, 2538 CrossRef CAS.
- J. Du, L. Jiao, Q. Wu, Y. Liu, Y. Zhao, L. Guo, Y. Wang and H. Yuan, Electrochim. Acta, 2013, 103, 219 CrossRef CAS.
- P. Barpanda, G. Rousse, T. Ye, C. D. Ling, Z. Mohamed, Y. Klein and A. Yamada, Inorg. Chem., 2013, 52, 3334 CrossRef CAS PubMed.
- M. Tatsumisago, Y. Kowada and T. Minami, J. Non-Cryst. Solids, 1992, 150, 207 CrossRef CAS.
- A. C. Larson and R. B. Von Dreele, General Structure Analysis Software (GSAS), Los Alasmos National Laboratory Report LAUR, 2004, pp. 86–748 Search PubMed.
- K. Momma and F. Izumi, J. Appl. Crystallogr., 2011, 44, 1272 CrossRef CAS.
- J. Mendham, R. C. Denney, J. D. Barnes and M. J. K. Thomas, Vogel's Text Book of Quantitative Chemical Analysis, 6th edn, Pearson Education Asia, Singapore, 2002 Search PubMed.
- D. P. Almond and A. R. West, Nature, 1983, 306, 456 CrossRef CAS.
- M. M. Ahmad, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 72, 174303 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra00014e |
| This journal is © The Royal Society of Chemistry 2014 |



