A new pathway for the formation of radial nematic droplets within a lipid-laden aqueous-liquid crystal interface†
Abstract
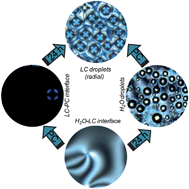
A new pathway for the formation of liquid crystal (LC) droplets with radial LC ordering in the presence of surfactants and lipids is reported. This study also shows that the response of these droplets due to the interactions between an enzyme and the topological defects in the LC may be exploited in applications such as sensing.

 Please wait while we load your content...
Please wait while we load your content...