A specially designed Li–H2O2 semi-fuel cell: A potential choice for electric vehicle propulsion†
Kai Liu,
Chang-An Wang* and
Jiang-Tao Ma
State Key Lab of New Ceramics and Fine Processing, School of Materials Science and Engineering, Tsinghua University, Beijing 100084, P. R. China. E-mail: wangca@mail.tsinghua.edu.cn
First published on 5th March 2014
Abstract
A specially designed Li–H2O2 semi-fuel cell based on hybrid electrolytes is proposed. It is a combination of fuel cell and lithium battery. Five “mechanical charge–discharge” cycles (800 hours in total) of the Li–H2O2 semi-fuel cell were conducted. The cell exhibits fast mechanical rechargeability, good stability and high lithium utilization. Output power of the Li–H2O2 semi-fuel cell can be flexibly adjusted by changing H2O2 concentration.
The concern over energy exhaustion and environment pollution has made electric vehicles (EVs) more and more attractive. Driven by electric power transformed from water, wind, nuclear, geothermal and solar energy, EVs can reduce emission of greenhouse gas and reliance on fossil fuels. Better EVs require higher stability, larger capacity, shorter recharge time and lower-cost batteries. Recently, Li–O2 battery has received considerable attention due to super-high theoretical specific energy, and it is considered as the power battery technology of the next generation.1–3
However, at the present stage, rechargeable Li–O2 battery still suffers from several problems:4–7 (1) relative poor cycle performance. On the one hand, there is a significant capacity fade during charge–discharge cycles caused by electrical passivation at the cathode, growth of lithium dendrite and decomposition of electrolyte.8,9 On the other hand, the voltage efficiency is relatively low. (2) Limited practical cell capacity. The cell capacity is limited by the capacity of porous air cathode,10 which is much lower than that of lithium anode (3862 mA h g−1 theoretically) due to clogging. (3) Long recharge time, which is as long as that of traditional batteries. Li–O2 battery with organic electrolyte exhibits a relatively good charge–discharge cycle performance, but clogging often occurs; using aqueous electrolyte can alleviate the clogging problem, unfortunately the charging behaviour of Li–O2 battery with aqueous electrolyte is undetermined.11 These problems have plagued efforts to develop Li–O2 battery technology for practical use.
Since it is difficult to solve the problems, we may avoid them. As charge–discharge cycle performance of Li–O2 battery is poor, and recharge process takes a long time and causes growth of lithium dendrite and exacerbates decomposition of the electrolyte, it may be better to replace traditional recharge by mechanical recharge. Keeping this in mind, we paid attention to the mechanical rechargeable metal–O2 and metal–H2O2 fuel/semi-fuel cells,12–15 which use reactive metal (Al, Mg and Zn as usual) as the anode (i.e., fuel) and O2 or H2O2 as the oxidant, and convert stored chemical energy to electrical energy.
Among all the metal anode fuel/semi-fuel cells, Li–H2O2 semi-fuel cell may be a good choice for EVs in the future, for lithium is the most negative metal while possessing a super-high capacity. Li–H2O2 semi-fuel cell is a combination of lithium battery and fuel cell, enjoying the advantages of high theoretical specific energy, large capacity, and fast mechanical rechargeability. The application of H2O2 will not contaminate the environment, furthermore, H2O2 is easy to be added into the liquid electrolyte and its concentration can be varied in a wide range as H2O2 and water are completely miscible. These properties make it convenient to adjust the cell voltage, i.e., output power, of the Li–H2O2 semi-fuel cell by changing H2O2 concentration in the catholyte.
Li–H2O2 semi-fuel cell had been reported by Gautum Agarwal in 1999.16 In their cell, a 3% H2O2 solution was used as electrolyte and lithium was immersed in the solution. Unfortunately, the cell failed to discharge, and caught fire immediately. To the best of our knowledge, there have been no more reports about Li–H2O2 semi-fuel cell to date. To operate a Li–H2O2 semi-fuel cell stably and efficiently, the lithium anode must be strictly protected from any contact with H2O2, H2O or air.
In order to ensure good protection of lithium anode in the Li–H2O2 semi-fuel cell, hybrid electrolyte type cell17–25 is preferred, and to enable fast mechanical rechargeability, the cell structure should be specially designed. In our study, a hybrid electrolyte type Li–H2O2 semi-fuel cell with novel cell structure was proposed, and the cell performance was investigated.
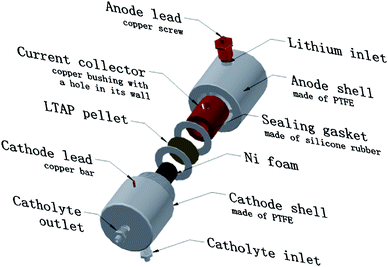
Fig. 1 shows the cell structure of our Li–H2O2 semi-fuel cell. In the assembled cell, the Ni foam was rolled up and placed inside the cathode shell. The current collector was wedged in the anode shell with its hole aligned to the hole in the anode shell so that a lithium inlet was formed. The threads of cathode shell engaged with the mating threads of anode shell were tightened to compress the gaskets and LTAP pellet (purchased from Ohara Inc., Japan) between the two shells. The space inside the tightened shells was divided into two chambers (cathode chamber and anode chamber) by the gaskets, LTAP pellet and a small amount of epoxy glues, and only Li+, but no liquid or gas species, could pass through. The assembled semi-fuel cell received fuel, lithium, in an argon protected glove box. Small lithium particles were put into the anode chamber through the lithium inlet and adhered to the inner wall of the current collector; a few drops of organic electrolyte (1 M LiPF6 in ethylene carbonate and diethyl carbonate (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v)) were added as well. Then the copper screw was tightened to keep a good electrical contact with the current collector and sealed lithium inside the anode chamber. The fuelled up cell can be removed from the glove box and discharged in ambient air. When the discharge was finished, we moved the cell into the argon protected glove box, twisted the copper screw off, refuelled the cell by adding certain amounts of fresh lithium particles, tightened the copper screw, then mechanical recharge was finished. This entire recharge process took less than 10 minutes.
1 v/v)) were added as well. Then the copper screw was tightened to keep a good electrical contact with the current collector and sealed lithium inside the anode chamber. The fuelled up cell can be removed from the glove box and discharged in ambient air. When the discharge was finished, we moved the cell into the argon protected glove box, twisted the copper screw off, refuelled the cell by adding certain amounts of fresh lithium particles, tightened the copper screw, then mechanical recharge was finished. This entire recharge process took less than 10 minutes.
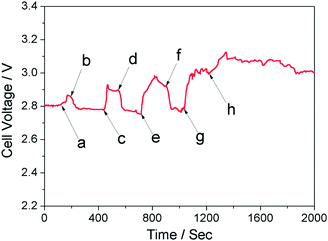
Fig. 2 shows discharge curves of the tests. During the 800 h tests, the semi-fuel cell was mechanically recharged 5 times. Hence the fast mechanical rechargeability of the designed Li–H2O2 semi-fuel cell was confirmed. The average discharge voltage was 2.7 V, which was at the same level as the voltages of most reported Li–O2 batteries.26–30 Performance of the Li–H2O2 semi-fuel cell could be further improved by employing more advanced positive electrode.12,31,32 The discharge voltage plateau in each voltage vs. discharge time curves suggested a good stability of our semi-fuel cell.
The open circuit voltage (OCV) was 3.0 V, which was much lower than the theoretical OCV of 4.83 V given by eqn (1) and (2)
| (−): Li → Li+ + e− (φ = −3.05 V) | (1) |
| (+): H2O2 + 2H++ 2e− → 2H2O (φ = +1.78 V) | (2) |
According to previous reports,33,34 in neutral solution such as the catholyte in our case, reactions like eqn (3), (4) and (5) show may occur at the cathode as well (O2 was generated from the chemical decomposition of H2O2)
| H2O2 + 2e− → 2OH− (φ = +0.878 V) | (3) |
| O2 + 2H++ 2e− → H2O2 (φ = +0.695 V) | (4) |
| O2 + 2H2O + 4e− → 4OH− (φ = +0.401 V) | (5) |
We investigated the open circuit potential (OCP) of the cathode, the observed OCP was only ∼0.52 V (Fig. S1†), which was much lower than its theoretical value of 1.78 V (see eqn (2)). This result indicates that the reaction couple at the electrode was not solely H2O2/H2O as summarized in eqn (2), it was more likely a mixture of H2O2/H2O, H2O2/OH−, O2/H2O2 and O2/OH−. These side reactions in cathode resulted in a low OCV. The reaction in cathode is quite complicated, further research is needed to clarify the mechanism.
The gap between OCV and discharge voltage became larger in the third and fourth tests, and discharge voltage slightly decreased with the increase in the discharge depth. This phenomenon could be attributed to the increase in internal resistance of the semi-fuel cell. As LTAP pellet was stable in catholyte, the rise of the internal resistance was probably caused by the degeneration of Ni foam (Fig. S2 and S4†). Actually, after replacing the aged Ni foam with new Ni foam (it is easy to replace through the catholyte outlet without disassembling the cell), the difference between OCV and discharge voltage in the fifth test decreased.
The capacity of rechargeable Li–O2 battery is fixed and confined by the amount of lithium sealed inside the battery. However, the capacity of the Li–H2O2 semi-fuel cell increases with the amount of lithium added (see Table 1). This is as simple as the fact that the distance a car can cover depends on the amount of gasoline in the tank. Actually, mechanical recharge process was almost as fast as the refuel process of a gasoline engine.
| Weight of lithium (mg) | Cell capacity (mA h) | Specific capacity (mA h g−1 Li) | Lithium utilization (%) |
|---|---|---|---|
| 2.9 | 9.1 | 3138 | 81.3 |
| 10.4 | 31.1 | 2990 | 77.4 |
| 4.2 | 13.2 | 3143 | 81.5 |
| 3.6 | 11.6 | 3222 | 83.5 |
| 3.1 | 10.1 | 3258 | 84.4 |
Utilization of the fuel, lithium, was calculated by comparing practical specific capacity (based on the mass of lithium) to theoretical one (3862 mA h g−1). Table 1 lists the lithium utilization in Li–H2O2 semi-fuel cell. It can be found that high average lithium utilization of 81.6% was gained, and it was almost independent of the mass of lithium added to the cell. More importantly, the specific capacity did not decrease at least during the first five “mechanical charge–discharge” cycles, which lasted for 34 days. High lithium utilization and relative stable specific capacity indicate that lithium in the cell had been well protected, so the corrosion reaction of lithium was very small and capacity fade caused by electrolyte decomposition and lithium dendrite formation was negligible.
H2O2 dissolved in LiCl solution acts as an active cathode substance, and its concentration affects the cell performance significantly. Fig. 3 shows the effect the H2O2 concentration on OCV of Li–H2O2 semi-fuel cell. It can be seen that the OCV of the cell increased with H2O2 concentration. The cell could be regarded as a Li–water/dissolved oxygen semi-fuel cell without H2O2 in the catholyte, and the OCV was lower than 2.75 V. When adding a small amount of H2O2 to the catholyte to get a low H2O2 concentration of 0.05 M, an increment of more than 100 mV in OCV was observed. When H2O2 concentration increased to 2.0 M, OCV reached 3.24 V, 500 mV higher than the initial one.
According to Nernst equation, it is reasonable to suggest a relationship between OCV and H2O2 concentration as:
OCV = A + B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c(H2O2) c(H2O2)
| (6) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c(H2O2). As can be seen, the measured values match well with the fitted line. Eqn (6) is only an empirical equation, nevertheless, it is still meaningful. According to eqn (6), OCV of the semi-fuel cell, as well as the cell voltage and output power, could be conveniently adjusted by adjusting H2O2 concentration.
c(H2O2). As can be seen, the measured values match well with the fitted line. Eqn (6) is only an empirical equation, nevertheless, it is still meaningful. According to eqn (6), OCV of the semi-fuel cell, as well as the cell voltage and output power, could be conveniently adjusted by adjusting H2O2 concentration.
To verify this inference, various amounts of 30% H2O2 solution was injected directly into the 5 mL cathode chamber while the semi-fuel cell was discharged at a constant current of 0.1 mA. As Fig. 4 shows, when stopping the flow of catholyte and injecting H2O2 solution into the cathode chamber, the H2O2 concentration inside the chamber increased, and the cell voltage increased consequently. The amount of H2O2 solution injected into the chamber was positively related to, but not directly proportional to, the increment of cell voltage, which generally conformed to eqn (6). When starting the flow of catholyte, the increased cell voltage dropped quickly, for the catholyte with high H2O2 concentration inside the cathode chamber was diluted. During the experiment, discharge current (I) was kept constant, so output power (P = I × U) of the Li–H2O2 semi-fuel cell changed as cell voltage (U) changed.
Catholyte flow rate also affects cell voltage, as shown in Fig. 5. When the catholyte did not flow, cell voltage decreased while discharging, which could be attributed to continuously decrease of H2O2 concentration around the cathode. With catholyte flow, there was a supplement of H2O2, so that H2O2 concentration around the cathode was stable, and discharge voltage was kept relative stable consequently. Furthermore, the higher the flow rate, the higher the discharge voltage, but there was a limit, and too high a flow rate, such as 20 mL min−1 in our experiment, would make the discharge voltage unstable. This results is consistent with ref. 35.
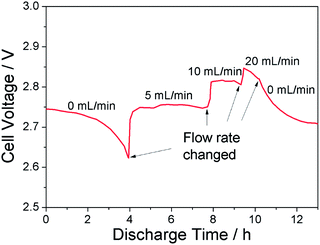 | ||
Fig. 5 Effects of the flow rate on the cell voltage. Anolyte: 1.0 M LiPF6 in EC–DEC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by volume). Catholyte: 0.1 M H2O2 + 2 M LiCl. Current: 0.1 mA. Operation temperature: 25 °C. 1 by volume). Catholyte: 0.1 M H2O2 + 2 M LiCl. Current: 0.1 mA. Operation temperature: 25 °C. | ||
EV needs different power in different operating conditions. For Li–H2O2 semi-fuel cell, output power can be flexibly adjusted to meet EV's different requirement of power by changing H2O2 concentration and catholyte flow rate. When low power is needed, operation on the semi-fuel cell is to keep the flow of catholyte, in which H2O2 concentration is relatively low; when high power is needed, stop the flow of catholyte, and inject a certain amount of H2O2 into the cathode chamber. After the requirement of high power is satisfied, restart the flow of the catholyte to dilute H2O2 inside the cathode chamber to keep chemical decomposition of H2O2 at a low level, for decomposition rate of H2O2 increases with H2O2 concentration.32 To confine H2O2 concentration in the catholyte, we can increase output power by increasing catholyte flow rate instead of adding H2O2.
The results suggested good stability and high lithium utilization of the specially designed Li–H2O2 semi-fuel cell. Besides, since Li–H2O2 semi-fuel cell is a combination of Li battery and fuel cell, it has advantages of both:
(1) High cell voltage and energy density. The molar mass and electrode potential of H2O2 is almost the same as that of O2. As a result, the cell voltage and energy density (based on the mass of active materials on both electrodes) of Li–H2O2 semi-fuel cell is almost the same as that of Li–O2 battery. Actually, the present semi-fuel cell exhibited a cell performance similar to Li–O2 battery.
(2) Fast mechanical rechargeability. The special cell structure allowed the semi-fuel cell to get mechanically recharged, which took less than 10 minutes, almost as fast as the refuelling process of a gasoline engine. The mechanical recharge process could be even faster in the future, if conducted in situ by employing an argon protected pump gun to pump lithium particles into anode chamber, without detaching the cell from EV and moving it to an argon protected box.
(3) Large capacity. In the discharge tests, with more lithium stored in the anode chamber, the capacity of the semi-fuel cell became correspondingly larger. It depended on the maximum amount of lithium that can be stored in the anode chamber, or lithium storage tank outside the cell. Theoretically, the energy capacity can be as large as designed, and sustain EV drive for a long distance.
(4) Flexibility in adjusting output power. The cell voltage and output power was very sensitive to H2O2 concentration and catholyte flow rate. By injecting a certain amount of H2O2 into the cathode chamber and changing flow rate, the output power of the semi-fuel cell can be controlled to meet different power requirements of the EV.
Moreover, combined with a lithium recycle system, the fuel of Li–H2O2 semi-fuel cell, lithium, can be cyclically utilized,36 as Fig. 6 shows. The recharge processes in individual batteries, i.e., electrolysis of lithium salts producing lithium metal, are integrated and conducted in electrolysis plants instead, which might be meaningful in energy saving and efficiency enhancement. In the lithium recycle loop, energy (solar, wind, water, tide, nuclear energy and so on) supplied to the electrolysis plants finally drives cars, lithium just acts as an “energy carrier”. H2O2 is a common industrial product, and lithium resource is relatively rich on earth, and the method of producing Li from LiOH is well known, Li–H2O2 semi-fuel cell with a lithium recycle system may be a promising environmentally friendly power source for EVs in the future. At present, some problems remain. For example, the Li–H2O2 semi-fuel cell still suffers a large internal resistance, resulting in a poor high rate discharge performance (see Fig. S5†). Further research is required.
Conclusions
A hybrid electrolyte based Li–H2O2 semi-fuel cell with a specially designed cell structure was proposed. Five “mechanical recharge–discharge” cycles (800 hours in total) were conducted, and the effects of H2O2 concentration and catholyte flow rate on the cell performance were studied. The cell exhibited good stability, high lithium utilization, fast mechanical rechargeability and adjustability of capacity, cell voltage and output power. Considering all the features mentioned above, Li–H2O2 semi-fuel cell is a promising power battery technology and it provides a new choice for electric vehicle propulsion.Acknowledgements
The authors would like to thank the National Natural Science Foundation of China for financial support (NSFC-no. 51221291 and 51172119).Notes and references
- P. G. Bruce, S. A. Freunberger, L. J. Hardwick and J.-M. Tarascon, Nat. Mater., 2012, 11, 19 CrossRef CAS PubMed.
- M. Park, H. Sun, H. Lee, J. Lee and J. Cho, Adv. Energy Mater., 2012, 2, 180 Search PubMed.
- D. Capsoni, M. Bini, S. Ferrari, E. Quartarone and P. Mustarelli, J. Power Sources, 2012, 220, 253 CrossRef CAS PubMed.
- F. Li, T. Zhang and H. Zhou, Energy Environ. Sci., 2013, 6, 1125 CAS.
- N.-S. Choi, Z. Chen, S. A. Freunberger, X. Ji, Y.-K. Sun, K. Amine, G. Yushin, L. F. Nazar, J. Cho and P. G. Bruce, Angew. Chem., Int. Ed., 2012, 51, 9994 CrossRef CAS PubMed.
- R. Cao, J.-S. Lee, M. Liu and J. Cho, Adv. Energy Mater., 2012, 2, 816 CrossRef CAS.
- R. Padbury and X. Zhang, J. Power Sources, 2011, 196, 4436 CrossRef CAS PubMed.
- S. A. Freunberger, Y. Chen, Z. Peng, J. M. Griffin, L. J. Hardwick, F. Bardé, P. Novák and P. G. Bruce, J. Am. Chem. Soc., 2011, 133, 8040 CrossRef CAS PubMed.
- Y. Chen, S. A. Freunberger, Z. Peng, F. Bardé and P. G. Bruce, J. Am. Chem. Soc., 2012, 134, 7952 CrossRef CAS PubMed.
- I. C. Jang, Y. Hidaka and T. Ishihara, J. Power Sources, 2013, 244, 606 CrossRef CAS PubMed.
- J. Wang, Y. Li and X. Sun, Nano Energy, 2013, 2, 443 CrossRef CAS PubMed.
- C. J. Patrissi, R. R. Bessette, Y. K. Kim and C. R. Schumacher, J. Electrochem. Soc., 2008, 155, B558 CrossRef CAS PubMed.
- D. J. Brodrecht and J. J. Rusek, Appl. Energy, 2003, 74, 113 CrossRef CAS.
- S. Zhang, J. Jiang and D. Zhou, Int. J. Energy Res., 2012, 36, 953 CrossRef CAS.
- P. Sapkota and H. J. Kim, Ind. Eng. Chem. Res., 2009, 15, 445 CrossRef CAS PubMed.
- G. Agarwal, J. Propulsion, 1999, 16, 367 CrossRef PubMed.
- Y. Wang, P. He and H. Zhou, Energy Environ. Sci., 2010, 3, 1515 CAS.
- H. Zhou, Y. Wang, H. Li and P. He, ChemSusChem, 2010, 3, 1009 CrossRef CAS PubMed.
- Y. Wang and H. Zhou, J. Power Sources, 2010, 195, 358 CrossRef CAS PubMed.
- E. Yoo, J. Nakamura and H. Zhou, Energy Environ. Sci., 2012, 5, 6928 CAS.
- T. Zhang, N. Imanishi, Y. Shimonishi, A. Hirano, Y. Takeda, O. Yamamoto and N. Sammes, Chem. Commun., 2010, 46, 1661 RSC.
- L. Li, X. Zhao and A. Manthiram, Electrochem. Commun., 2012, 14, 78 CrossRef CAS PubMed.
- L. Li and A. Manthiram, J. Mater. Chem. A, 2013, 1, 5121 CAS.
- Y. Li, K. Huang and Y. Xing, Electrochim. Acta, 2012, 81, 20 CrossRef CAS PubMed.
- Y. Li, Z. Huang, K. Huang, D. Carnahan and Y. Xing, Energy Environ. Sci., 2013, 6, 3339 CAS.
- D. Xu, Z.-L. Wang, J.-J. Xu, L.-L. Zhang and X.-B. Zhang, Chem. Commun., 2012, 48, 6948 RSC.
- Z. Guo, G. Zhu, Z. Qiu, Y. Wang and Y. Xia, Electrochem. Commun., 2012, 25, 26 CrossRef CAS PubMed.
- Y.-C. Lu, B. M. Gallant, D. G. Kwabi, J. R. Harding, R. R. Mitchell, M. S. Whittingham and S.-H. Yang, Energy Environ. Sci., 2013, 6, 750 CAS.
- W. Zhang, Y. Zeng, C. Xu, H. Tan, W. Liu, J. Zhu, N. Xiao, H. H. Hng, J. Ma, H. E. Hoster, R. Yazami and Q. Yan, RSC Adv., 2012, 2, 8508 RSC.
- H.-G. Jung, J. Hassoun, J.-B. Park, Y.-K. Sun and B. Scrosati, Nat. Chem., 2012, 4, 579 CrossRef CAS PubMed.
- W. Yang, S. Yang, W. Sun, G. Sun and Q. Xin, Electrochim. Acta, 2006, 52, 9 CrossRef CAS PubMed.
- C. Shu, E. Wang, L. Jiang, Q. Tang and G. Sun, J. Power Sources, 2012, 208, 159 CrossRef CAS PubMed.
- X. Jiang, D. X. Cao, Y. Liu, G. L. Wang, J. L. Yin, Q. Wen and Y. Y. Gao, J. Electroanal. Chem., 2011, 658, 46 CrossRef PubMed.
- Y. Wang, Z. Guo and Y. Xia, Adv. Energy Mater., 2013, 3, 713 CrossRef CAS.
- T. Lei, Y. Tian, G. Wang, J. Yin, Y. Gao, Q. Wen and D. Cao, Fuel Cells, 2011, 11, 431 CrossRef CAS.
- P. He, Y. Wang and H. Zhou, Electrochem. Commun., 2010, 12, 1686 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Open circuit potential (OCP) curves for cathode (H2O2); simple derivation from Nernst equation to eqn (5); picture of the LTAP pellet; SEM images and XRD patterns of the LTAP pellets before and after use; SEM images and EDS results of Ni foam before and after use; detailed information about LTAP pellet and Ni foam; polarization curve of the Li–H2O2 semi-fuel cell; picture of the Li–H2O2 semi-fuel cell test set-up. See DOI: 10.1039/c3ra47616b |
| This journal is © The Royal Society of Chemistry 2014 |