Photomechanical bending of linear azobenzene polymer†
Abstract
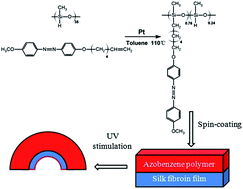
We firstly report a novel strategy for the preparation of rapid and reversible photo-driven actuators consisting of an active linear azobenzene polymer layer and a passive silk fibroin substrate, avoiding the need for oriented azobenzene liquid crystalline elastomers (LCEs) that have been used until now, just through depositing linear azobenzene polymer on the top of silk fibroin film. The unimorph actuators can show uniquely different bending properties. Moreover, the response speed of the unimorph actuator is on the level of a few hundreds of milliseconds. The bending angle can be well controlled either by changing the UV light intensity or by altering the thickness ratio of the two composed layers. Additionally, complex and attractive arm-like movements are successfully achieved. We believe that the proposed unimorph actuators will pave the way for designing complicated and programmed artificial muscles.

 Please wait while we load your content...
Please wait while we load your content...