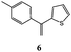
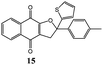
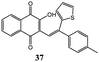
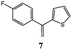
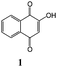
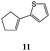
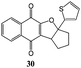
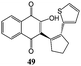
Synthesis of dihydrofuro- and C-alkenylated naphthoquinones catalyzed by manganese(III) acetate†
Oğuzhan Alagöz*ad,
Mehmet Yılmazb,
A. Tarık Pekelc,
Claudia Graiffd and
Raimondo Maggid
aAfyon Kocatepe Üniversitesi, Kimya Mühendisliği Bölümü, 03200 Afyonkarahisar, Turkey. E-mail: oalagoz@aku.edu.tr
bKocaeli Üniversitesi, Kimya Bölümü, 41380 Kocaeli, Turkey
cAnkara Üniversitesi, Kimya Bölümü, 06100 Ankara, Turkey
dDipartimento di Chimica, Università di Parma, I-43124 Parma, Italy
First published on 7th March 2014
Abstract
The easily synthesis of dihydrofuronaphthoquinones and C-alkenylated naphthoquinones has been investigated through the radical oxidative cyclization of 2-hydroxy-1,4-naphthoquinones and thiophene or furan substituted alkenes catalyzed by manganese(III) acetate. The corresponding heteroaryl substituted dihydrofurans and naphthoquinones have been isolated in good yields.
Introduction
Free radical reactions using transition metal salts, such as manganese, cerium and cobalt, for the formation of carbon–carbon bonds have become more and more important in organic syntheses during the last decades, mainly because of their selective, specific, and mild reaction facilities.1 One of the well-known examples of this application is the manganese(III) acetate mediated free radical reaction. This reaction has emerged as an important synthetic method for the formation of highly functionalized products such as furans,2 dihydrofurans,3 lactones,4 lactams,5 and natural products.6Compounds containing the quinone moiety represents an important class of biologically active molecules which are widespread in nature.7 Many natural and synthetic naphthoquinones are known as potential antitumor,8 tripanocidal,9 molluscicidal,10 leishmanicidal,11 antibacterial12 and antitubercular13 agents.
Dihydrofuro- and C-alkenylated naphthoquinones have recently gained considerable interest due to their useful application in the synthesis of a huge variety of valuable compounds.1,14 The present study reports the easily synthesis of these compounds by using Mn(OAc)3 as catalyst.
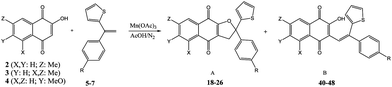
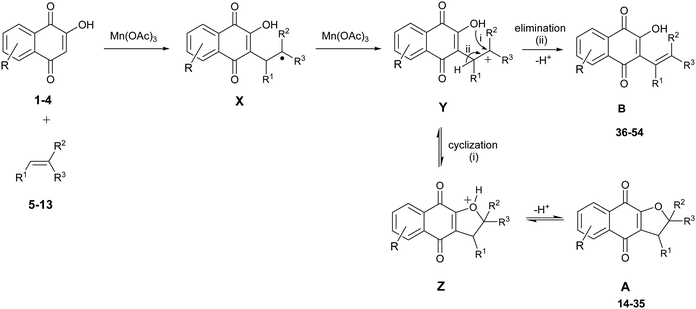
On the basis of these considerations, in the present contribution we have evaluated the possibility to develop the reaction between 2-hydroxy-1,4-naphthoquinones (1–4) and heteroaromatic substituted alkenes (5–13) in the presence of Mn(OAc)3 as catalyst and in acetic acid as medium.
Results and discussion
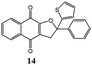
In order to find the best reaction conditions, at first we have analyzed three parameters, namely the reagent molar ratio, the reaction temperature and the reaction time, by using 2-hydroxy-1,4-naphthoquinone 1 and 2-(1-phenylethenyl)thiophene 5 as model reagents. Concerning the first parameter examined, after having performed the reaction with different 1/5/Mn(OAc)3 molar ratios in acetic acid for 10 minutes at 65 °C, the best yield in product 14 (77%) was obtained by using 1/1.25/2.5 molar ratio.Then the variation of the yield with respect to reaction temperature and time was studied (Table 1); the best result in term of yield and selectivity in dihydrofuronaphthoquinone 14 was obtained at 65 °C for 5 minutes (Table 1, entry 2). A further increase of the temperature (up to 95 °C) or of the reaction time (up to 60 minutes) resulted in lower yield and selectivity values (Table 1, entries 7–9 and 3–5 respectively).
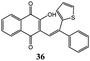
Interestingly, by carrying out the reaction at 65 °C for longer reaction times (24 hours), the C-alkenylated naphthoquinone 36, deriving from dihydrofuronaphthoquinone 14, was formed in 60% yield with and 98% selectivity (Table 1, entry 6). The same good result can be obtained by increasing the temperature to 95 °C and the reaction time to 2 hours (Table 1, entry 12).
By using 2-hydroxy-1,4-naphthoquinone 1 the process was then extended to various 1,1-disubstitued alkenes 5–8 by carrying out the reaction in acetic acid in the presence of Mn(OAc)3 at 65 °C for 10 minutes; results are shown in Table 2.
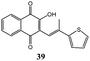
Under these conditions dihydrofuronaphthoquinones 14–17 were isolated in good yields (up to 89%) in a very short reaction time. The highest yields were observed by using electron rich 1,1-disubstituted alkenes, probably because the stability of carbocation intermediate is increased by the presence of electron donating substituents. The yields of products 36–39 were lower (9–11%), but they can be increased up to ∼75% by increasing the reaction time to 24 hours (Table 3). Interestingly, 1H NMR spectra of products 36–39 showed that they are obtained as cis–trans (Z–E) mixtures except for product 39 that was obtained as a single E isomer; this is probably due to the larger size of the thiophene group with respect to the methyl one.
The reaction was also extended to various 2-hydroxy-1,4-naphthoquinones 2–4 with 1,1-disubstituted alkenes 5–7 (Table 4). After 5 minutes the dihydrofuronaphthoquinones 18–26 are the sole reaction products and can be isolated in 64–86% yield; the best results are gained when a fluorine atom is present on the aromatic ring of the alkene.
| Entry | X | Y | Z | R | Time (min) | A (%) | B (%) | B Z-E ratio |
|---|---|---|---|---|---|---|---|---|
| 1 | H | H | Me | H | 5 | 18 (66) | — | — |
| 2 | Me | H | Me | H | 5 | 19 (64) | — | — |
| 3 | H | MeO | H | H | 5 | 20 (72) | — | — |
| 4 | H | H | Me | Me | 5 | 21 (73) | — | — |
| 5 | Me | H | Me | Me | 5 | 22 (69) | — | — |
| 6 | H | MeO | H | Me | 5 | 23 (81) | — | — |
| 7 | H | H | Me | F | 5 | 24 (79) | — | — |
| 8 | Me | H | Me | F | 5 | 25 (73) | — | — |
| 9 | H | MeO | H | F | 5 | 26 (86) | — | — |
| 10 | H | H | Me | H | 24 h | 18 (<1) | 40 (47) | 1/2.3 |
| 11 | Me | H | Me | H | 24 h | 19 (<1) | 41 (57) | 1/2.5 |
| 12 | H | MeO | H | H | 24 h | 20 (<1) | 42 (69) | 1/2 |
| 13 | H | H | Me | Me | 24 h | 21 (<1) | 43 (49) | 1/2.2 |
| 14 | Me | H | Me | Me | 24 h | 22 (<1) | 44 (60) | 1/2.3 |
| 15 | H | MeO | H | Me | 24 h | 23 (<1) | 45 (77) | 1/2 |
| 16 | H | H | Me | F | 24 h | 24 (<1) | 46 (70) | 1/3 |
| 17 | Me | H | Me | F | 24 h | 25 (<1) | 47 (69) | 1/3 |
| 18 | H | MeO | H | F | 24 h | 26 (<1) | 48 (80) | 1/3 |
By performing the reaction for 24 hours, the corresponding C-alkenylated naphthoquinones 40–48 can also be isolated in 47–80% yield and <99% selectivity.
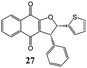
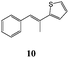
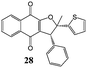
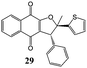
2-Hydroxy-1,4-naphthoquinone (1) was also reacted with 2-[(E)-2-phenylethenyl]thiophene (9) and only dihydrofuran product 27 was obtained in 56% yield (Table 5). From the coupling constant value between H4 and H5 (J = 6.0 Hz) it was deduced that the phenyl and 2-thienyl moieties are in the cis configuration in the 4,5-dihydro-4-phenyl-5-(2-thienyl)furan.15 On the contrary, two different dihydrofurans were obtained by reaction of 2-hydroxy-1,4-naphthoquinone (1) with 2-(1-methyl-2-phenyl-vinyl)-thiophene (10) (Table 5); in this case too the alkenyl naphthoquinone was not isolated. The determination of dihydrofuran structures (28,29) was realized by HSQC measurement: the proton at ∼5 ppm was determined to be on C(4). This indicates that the phenyl group is on C(4) and the 2-thienyl and methyl groups are on C(5) of the dihydrofuran moiety.
Substituted cyclic alkenes 11 and 12 were also utilized in the reaction with 2-hydroxy-1,4-naphthoquinone 1. In this case dihydronaphthoquinones 30 and 31 and alkenyl naphthoquinones 49 and 50 were isolated as single trans isomers (Table 6).
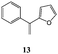
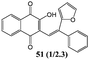
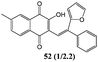
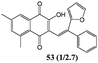
In order to evaluate the influence on the yield of the presence of thiophene and furane rings on the alkene moiety, the reactions between 2-hydroxy-1,4-naphthoquinones 1–4 and 2-(1-phenylvinyl)furane 13 were performed. Dihydrofurans 32–35 were obtained in moderate yields (54–62%), whereas alkenyl naphthoquinones 51–54 were formed as a isomeric mixture (∼1/2.5) in >50% yield (Table 7). In must be underlined that the yields obtained with the thiophene substituted alkenes are higher than those obtained with the furan substituted ones.
| Alkene | 2-Hydroxy naphthoquinone | Products | Yield, % Aa (Bb) | |
|---|---|---|---|---|
| Cyclization A | Elimination B (E/Z ratio) | |||
| a Yields for compounds A (32–35) were calculated from the results of 10 minutes reaction.b Yields for compounds B (51–54) were calculated from the results of 24 hours reaction. | ||||
 |
1 | 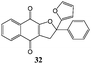 |
 |
62 (55) |
| 2 | 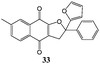 |
 |
57 (50) | |
| 3 | 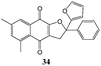 |
 |
54 (53) | |
| 4 | 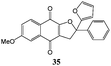 |
 |
59 (57) | |
On the basis of the above reported results, a plausible mechanism for the formation of dihydrofuran and elimination products is shown in Scheme 1. According to this mechanism, the reaction of 2-hydroxy-1,4-naphthoquinone 1 with alkene 2 catalyzed by Mn(OAc)3 produces a carbon-radical X. This radical X is in turn oxidized to the carbocation Y by one equivalent of Mn(OAc)3, followed by cyclization and proton elimination to give dihydrofuronaphtho-4,9-diones A. Concerning the formation of 3-alkenyl-2-hydroxy-1,4-naphthoquinone B, it can be obtained either through H+ elimination from carbocation Y or by ring opening of dihydrofuronaphtho-4,9-diones A and then H+ elimination.
Dihydrofuronaphthoquinone is the sole product at beginning of the reactions (ca. 5 min); however C-alkenylated product is formed at prolonged reaction times and at high temperatures. Therefore the formation of dihydrofuronaphthoquinones is an equilibrium: by increasing reaction temperature and time, the equilibrium can be shifted through the formation of the C-alkenylated product.
Finally, it must be underlined that in a previous work,16 only the dihydrofuran product was obtained as a result of the reaction of 1 with 1,1-diphenyl ethylene; indeed in the present work both dihydrofuran and C-alkenylated (elimination) products were synthesized in the reactions of 1–4 with heteroaromatic substituted alkenes (5–13). On the basis of 1H NMR spectra of cyclization products A, it has been possible to identify a cis–trans isomeric mixture; one of these isomers was finally confirmed by X-ray crystallography and an ORTEP view is reported in Fig. 1 together with the atomic labeling scheme and a list of the most important bond distances and angles.
 | ||
| Fig. 1 ORTEP view of compound 36 together with the atomic labeling scheme. Ellipsoids are drawn at their 50% probability level. Most important bond distances (Å) and angles (°): C1–O1 1.336(2), C2–O2 1.221(2), C9–O3 1.202(3), C10–C11 1.477(3), C11–C12 1.318(3), C12–C17 1.510(3), C12–C13 1.511(3); C12–C11–C10 127.4(2), C11–C12–C17124.32(19), C11–C12–C13 120.4(2), C17–C12–C13 115.20(17).† | ||
In the molecular structure of compound 36 the alkenyl group defined by atoms C10, C11, H11, C12, C13, C17 is planar. The mean planes of the phenylic, thiophenic and naphthoquinone substituents form dihedral angles of 44.15(4)°, 38.34(4)° and 70.50(5)° respectively with the alkenyl mean plane. In the crystal packing of compound 36, two molecules are linked by hydrogen bonds between the hydroxylic and the quinonic oxygen atoms forming a dimer. In particular the O1⋯O2 distance is 2.900(4) Å and O1–H1⋯O2 angle is 148.11(3)°.
Conclusions
We have described a simple, convenient and efficient preparation of polyfunctionalized dihydrofuronaphtho-4,9-diones (14–35) and 3-alkenyl-2-hydroxy-1,4-naphthoquinones (36–54). The simplicity of the procedure, the mildness of the reaction conditions and the good yields represent significant improvement. Effects of temperature, reaction time and substituent over product formation were studied, and complementary studies are in progress in order to explain different product formation and to extend the reaction to other substrates.Experimental
2-Hydroxy-1,4-naphthoquinone 1 is an available commercial product and has been used in high purity. 2-Hydroxy-1,4-naphthoquinone derivatives 2–4 are synthesized using suitable starting materials.17,18 The conjugated alkenes 5–13 were prepared by dehydration reaction from the carbinole formed by Grignard reaction of arylmagnesium bromide and suitable carbonyl compounds.19 2-Isopropenylthiophene 8 was prepared by reaction of triphenylphosphoniummethyl bromide and 2-acetylthiophene in NaH–THF.20 All conjugated alkenes were freshly prepared before use in the radical cyclization reactions. Radical reactions were performed at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.25
1.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 molar ratio [naphthoquinone
2.5 molar ratio [naphthoquinone![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) alkene
alkene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn(OAc)3] under nitrogen atmosphere in AcOH. All compounds were purified by column chromatography or preparative TLC and were characterized by IR, 1H–13C NMR, mass spectra and microanalyses.
Mn(OAc)3] under nitrogen atmosphere in AcOH. All compounds were purified by column chromatography or preparative TLC and were characterized by IR, 1H–13C NMR, mass spectra and microanalyses.
Melting points were determined on an electrothermal capillary melting point apparatus. IR spectra (KBr disc, CHCl3) were obtained with a Matson 1000 FT-IR in the 400–4000 cm−1 range with 4 cm−1 resolution. 1H and 13C NMR spectra were recorded on a Bruker Avance DPX-400 MHz and Varian Mercury-400 High performance Digital FT-NMR spectrophotometers. Mass spectra were measured on a Waters 2695 Alliance Micromass ZQ (ESI+) LC/MS and Agilent Technologies 6890 N Network GC System spectrophotometer. Elemental analyses were performed on a VarioEL III CHNS instrument. Thin layer chromatography (TLC) was performed on Merck aluminium-packed silica gel plates. Purification of the products was performed by column chromatography on silica gel (Merck silica gel 60, 40–63 μm) or preparative TLC on silica gel of Merck (PF254–366nm).
Reaction of naphthoquinones with alkenes catalyzed by Mn(OAc)3
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 as eluent.
1 as eluent.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1651 (C
O), 1651 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1627, 1200 (C–O–C), 952, 717; 1H-NMR (400 MHz, CDCl3) δ 8.11 (dd, J = 7.6 and 1.6 Hz, 1H), 8.07 (dd, J = 7.6 and 1.6 Hz, 1H), 7.72 (td, J = 7.6 and 1.6 Hz, 1H), 7.68 (td, J = 7.6 and 1.6 Hz, 1H), 7.30 (dd, J = 6.8 and 1.6 Hz, 2H), 7.37 (m, 3H), 7.32 (dd, J = 5.2 and 1.2 Hz, 1H), 6.97 (dd, J = 3.6 and 1.2 Hz, 1H), 6.95 (dd, J = 5.2 and 3.6 Hz, 1H), 4.07 (d, J = 17.2 Hz, 1H), 3.84 (d, J = 17.2 Hz, 1H); 13C-NMR (100 MHz, CDCl3) δ 182.3, 177.8, 158.4, 147.5, 143.5, 134.4, 133.3, 133.2, 131.9, 128.9, 128.7, 127.1, 127.0, 126.8, 126.6, 126.3, 125.6, 123.6, 94.1, 43.5; LC/MS (ESI, m/z): 359.07 (MH+, 100). Anal. calcd for C22H14O3S: C, 73.72; H, 3.94; S, 8.95. Found: C, 73.82; H, 4.01; S, 8.92.
O), 1627, 1200 (C–O–C), 952, 717; 1H-NMR (400 MHz, CDCl3) δ 8.11 (dd, J = 7.6 and 1.6 Hz, 1H), 8.07 (dd, J = 7.6 and 1.6 Hz, 1H), 7.72 (td, J = 7.6 and 1.6 Hz, 1H), 7.68 (td, J = 7.6 and 1.6 Hz, 1H), 7.30 (dd, J = 6.8 and 1.6 Hz, 2H), 7.37 (m, 3H), 7.32 (dd, J = 5.2 and 1.2 Hz, 1H), 6.97 (dd, J = 3.6 and 1.2 Hz, 1H), 6.95 (dd, J = 5.2 and 3.6 Hz, 1H), 4.07 (d, J = 17.2 Hz, 1H), 3.84 (d, J = 17.2 Hz, 1H); 13C-NMR (100 MHz, CDCl3) δ 182.3, 177.8, 158.4, 147.5, 143.5, 134.4, 133.3, 133.2, 131.9, 128.9, 128.7, 127.1, 127.0, 126.8, 126.6, 126.3, 125.6, 123.6, 94.1, 43.5; LC/MS (ESI, m/z): 359.07 (MH+, 100). Anal. calcd for C22H14O3S: C, 73.72; H, 3.94; S, 8.95. Found: C, 73.82; H, 4.01; S, 8.92.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1646 (C
O), 1646 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1277 (C–O–C), 1027, 958, 698; 1H-NMR (400 MHz, CDCl3) δ 8.02 (d, J = 7.2 Hz, 2H) [8.08 (dd, J = 7.6 and 1.6 Hz, 2H)], 7.73 (td, J = 7.6 and 1.2 Hz, 1H) [7.69–7.78 (m, 2H)], 7.66 (td, J = 7.6 and 1.6 Hz, 1H), 7.48 (dd, J = 7.6 and 2.0 Hz, 1H), 7.37 (dd, J = 4.8 and 1.6 Hz, 1H), 7.25–7.31 (m, 4H), 6.99 (dd, J = 4.8 and 3.6 Hz, 1H), 6.92 (dd, J = 3.6 and 1.2 Hz, 1H) [6.93 (dd, J = 3.6 and 0.8 Hz, 1H)], 6.88 (s, 1H) [6.61 (s, 1H)], LC/MS, (ESI, m/z): 359.25 (MH+, 100). Anal. calcd for C22H14O3S: C, 73.72; H, 3.94; S, 8.95. Found: C, 73.55; H, 4.07; S, 9.03.
O), 1277 (C–O–C), 1027, 958, 698; 1H-NMR (400 MHz, CDCl3) δ 8.02 (d, J = 7.2 Hz, 2H) [8.08 (dd, J = 7.6 and 1.6 Hz, 2H)], 7.73 (td, J = 7.6 and 1.2 Hz, 1H) [7.69–7.78 (m, 2H)], 7.66 (td, J = 7.6 and 1.6 Hz, 1H), 7.48 (dd, J = 7.6 and 2.0 Hz, 1H), 7.37 (dd, J = 4.8 and 1.6 Hz, 1H), 7.25–7.31 (m, 4H), 6.99 (dd, J = 4.8 and 3.6 Hz, 1H), 6.92 (dd, J = 3.6 and 1.2 Hz, 1H) [6.93 (dd, J = 3.6 and 0.8 Hz, 1H)], 6.88 (s, 1H) [6.61 (s, 1H)], LC/MS, (ESI, m/z): 359.25 (MH+, 100). Anal. calcd for C22H14O3S: C, 73.72; H, 3.94; S, 8.95. Found: C, 73.55; H, 4.07; S, 9.03.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1657 (C
O), 1657 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1638, 1201 (C–O–C), 955, 703; 1H-NMR (400 MHz, CDCl3) δ 8.10 (dd, J = 7.6 and 1.6 Hz, 1H), 8.06 (dd, J = 7.6 and 1.6 Hz, 1H), 7.72 (td, J = 7.6 and 1.6 Hz, 1H), 7.68 (td, J = 7.6 and 1.6 Hz, 1H), 7.41 (d, J = 8 Hz, 2H), 7.31 (dd, J = 4.8 and 1.2 Hz, 1H), 7.19 (d, J = 8 Hz, 2H), 6.97 (dd, J = 3.6 and 1.2 Hz, 1H), 6.94 (dd, J = 4.8 and 3.6 Hz, 1H), 4.03 (d, J = 17.2 Hz, 1H), 3.82 (d, J = 17.2 Hz, 1H), 2.35 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 182.4, 177.8, 158.4, 147.7, 140.5, 138.6, 134.4, 133.3, 133.2, 131.9, 129.4, 127.0, 126.7, 126.6, 126.3, 125.6, 123.7, 94.1, 43.4, 21.3; GC/MS (m/z, %): 372.1 (M+, 100). Anal. calcd for C23H16O3S: C, 74.17; H, 4.33; S, 8.61. Found: C, 74.14; H, 4.30; S, 8.56.
O), 1638, 1201 (C–O–C), 955, 703; 1H-NMR (400 MHz, CDCl3) δ 8.10 (dd, J = 7.6 and 1.6 Hz, 1H), 8.06 (dd, J = 7.6 and 1.6 Hz, 1H), 7.72 (td, J = 7.6 and 1.6 Hz, 1H), 7.68 (td, J = 7.6 and 1.6 Hz, 1H), 7.41 (d, J = 8 Hz, 2H), 7.31 (dd, J = 4.8 and 1.2 Hz, 1H), 7.19 (d, J = 8 Hz, 2H), 6.97 (dd, J = 3.6 and 1.2 Hz, 1H), 6.94 (dd, J = 4.8 and 3.6 Hz, 1H), 4.03 (d, J = 17.2 Hz, 1H), 3.82 (d, J = 17.2 Hz, 1H), 2.35 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 182.4, 177.8, 158.4, 147.7, 140.5, 138.6, 134.4, 133.3, 133.2, 131.9, 129.4, 127.0, 126.7, 126.6, 126.3, 125.6, 123.7, 94.1, 43.4, 21.3; GC/MS (m/z, %): 372.1 (M+, 100). Anal. calcd for C23H16O3S: C, 74.17; H, 4.33; S, 8.61. Found: C, 74.14; H, 4.30; S, 8.56.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1652 (C
O), 1652 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1375, 1274 (C–O–C), 1022, 728, 666; 1H-NMR (400 MHz, CDCl3) δ 8.04 (t, J = 7.6 Hz, 2H) [8.07 (d, J = 7.6 Hz, 1H), 8.09 (d, J = 7.6 Hz, 1H)], 7.74 (t, J = 7.6 Hz, 1H) [7.69–7.77 (m, 2H)], 7.68 (t, J = 7.6 Hz, 1H), [7.37 (d, J = 8.0 Hz, 2H)], 7.30 (d, J = 4.8 Hz, 1H) [7.27 (d, J = 3.6 Hz, 1H)], 7.20 (d, J = 8.0 Hz, 2H), [7.17 (d, J = 8.0 Hz, 2H)], 7.06 (d, J = 8.0 Hz, 2H), 6.99 (dd, J = 4.8 ve 3.6 Hz, 1H), 6.95 (d, J = 3.6 Hz, 1H) [6.93 (d, J = 3.6 Hz, 1H)], 6.85 (s, 1H) [6.60 (s, 1H)], 2.32 (s, 3H) [2.40 (s, 3H)]; GC/MS (m/z, %): 372.1 (M+, 100). Anal. calcd for C23H16O3S: C, 74.17; H, 4.33; S, 8.61. Found: C, 74.29; H, 4.26; S, 8.73.
O), 1375, 1274 (C–O–C), 1022, 728, 666; 1H-NMR (400 MHz, CDCl3) δ 8.04 (t, J = 7.6 Hz, 2H) [8.07 (d, J = 7.6 Hz, 1H), 8.09 (d, J = 7.6 Hz, 1H)], 7.74 (t, J = 7.6 Hz, 1H) [7.69–7.77 (m, 2H)], 7.68 (t, J = 7.6 Hz, 1H), [7.37 (d, J = 8.0 Hz, 2H)], 7.30 (d, J = 4.8 Hz, 1H) [7.27 (d, J = 3.6 Hz, 1H)], 7.20 (d, J = 8.0 Hz, 2H), [7.17 (d, J = 8.0 Hz, 2H)], 7.06 (d, J = 8.0 Hz, 2H), 6.99 (dd, J = 4.8 ve 3.6 Hz, 1H), 6.95 (d, J = 3.6 Hz, 1H) [6.93 (d, J = 3.6 Hz, 1H)], 6.85 (s, 1H) [6.60 (s, 1H)], 2.32 (s, 3H) [2.40 (s, 3H)]; GC/MS (m/z, %): 372.1 (M+, 100). Anal. calcd for C23H16O3S: C, 74.17; H, 4.33; S, 8.61. Found: C, 74.29; H, 4.26; S, 8.73.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1655 (C
O), 1655 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1638, 1582, 1204 (C–O–C), 956, 713; 1H-NMR (400 MHz, CDCl3) δ 8.10 (dd, J = 7.6 and 1.6 Hz, 1H), 8.06 (dd, J = 7.6 and 1.6 Hz, 1H), 7.72 (td, J = 7.6 and 1.6 Hz, 1H), 7.68 (td, J = 7.6 and 1.6 Hz, 1H), 7.50 (ddd, J = 8.8 and 4.8 and 1.6 Hz, 2H), 7.33 (dd, J = 4.8 and 1.6 Hz, 1H), 7.07 (t, J = 8.8 Hz, 2H), 6.94–6.97 (m, 2H), 4.05 (d, J = 17.2 Hz, 1H), 3.78 (d, J = 17.2 Hz, 1H); 13C-NMR (100 MHz, CDCl3) δ 182.3, 177.7, 164.0, 161.6, 158.2, 147.3, 139.3, 134.4, 133.3, 131.9–133.2 (Cipso, i, J = 126.5 Hz), 127.6 (Cmeta, i, J = 8.4 Hz), 127.2, 127.1, 126.8, 126.6, 123.5, 115.7 (Cortho, i, J = 21.4 Hz), 93.6, 43.5; GC/MS, (m/z, %): 376.0 (M+, 100). Anal. calcd for C22H13FO3S: C, 70.20; H, 3.48; S, 8.52. Found: C, 70.17; H, 3.46; S, 8.48.
O), 1638, 1582, 1204 (C–O–C), 956, 713; 1H-NMR (400 MHz, CDCl3) δ 8.10 (dd, J = 7.6 and 1.6 Hz, 1H), 8.06 (dd, J = 7.6 and 1.6 Hz, 1H), 7.72 (td, J = 7.6 and 1.6 Hz, 1H), 7.68 (td, J = 7.6 and 1.6 Hz, 1H), 7.50 (ddd, J = 8.8 and 4.8 and 1.6 Hz, 2H), 7.33 (dd, J = 4.8 and 1.6 Hz, 1H), 7.07 (t, J = 8.8 Hz, 2H), 6.94–6.97 (m, 2H), 4.05 (d, J = 17.2 Hz, 1H), 3.78 (d, J = 17.2 Hz, 1H); 13C-NMR (100 MHz, CDCl3) δ 182.3, 177.7, 164.0, 161.6, 158.2, 147.3, 139.3, 134.4, 133.3, 131.9–133.2 (Cipso, i, J = 126.5 Hz), 127.6 (Cmeta, i, J = 8.4 Hz), 127.2, 127.1, 126.8, 126.6, 123.5, 115.7 (Cortho, i, J = 21.4 Hz), 93.6, 43.5; GC/MS, (m/z, %): 376.0 (M+, 100). Anal. calcd for C22H13FO3S: C, 70.20; H, 3.48; S, 8.52. Found: C, 70.17; H, 3.46; S, 8.48.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1590, 1276 (C–O–C), 1043, 730; 1H-NMR (400 MHz, CDCl3) δ 8.03 (d, J = 8.4 Hz, 2H) [8.08 (t, J = 8.4 Hz, 2H)], 7.74 (t, J = 7.6 Hz. 1H), 7.67 (t, J = 7.6 Hz. 1H) [7.65–7.77 (m, 2H)], [7.44 (td, J = 8.8 and 2.0 Hz, 2H)], 7.29 (m, 3H + [1H]), [7.05 (t, J = 8.8 Hz, 2H)], 6.90–7.00 (m, 5H + [3H]), 6.86 (s, 1H) [6.55 (s, 1H)]; GC/MS, (m/z, %): 376.0 (M+, 100). Anal. calcd for C22H13FO3S: C, 70.20; H, 3.48; S, 8.52. Found: C, 70.11; H, 3.48; S, 8.47.
O), 1590, 1276 (C–O–C), 1043, 730; 1H-NMR (400 MHz, CDCl3) δ 8.03 (d, J = 8.4 Hz, 2H) [8.08 (t, J = 8.4 Hz, 2H)], 7.74 (t, J = 7.6 Hz. 1H), 7.67 (t, J = 7.6 Hz. 1H) [7.65–7.77 (m, 2H)], [7.44 (td, J = 8.8 and 2.0 Hz, 2H)], 7.29 (m, 3H + [1H]), [7.05 (t, J = 8.8 Hz, 2H)], 6.90–7.00 (m, 5H + [3H]), 6.86 (s, 1H) [6.55 (s, 1H)]; GC/MS, (m/z, %): 376.0 (M+, 100). Anal. calcd for C22H13FO3S: C, 70.20; H, 3.48; S, 8.52. Found: C, 70.11; H, 3.48; S, 8.47.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1644 (C
O), 1644 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1626, 1204 (C–O–C), 955, 722; 1H-NMR (400 MHz, CDCl3) δ 8.11 (dd, J = 6.0 and 1.6 Hz, 1H), 8.08 (dd, J = 6.0 and 1.6 Hz, 1H), 7.73 (td, J = 7.6 and 1.6 Hz, 1H), 7.69 (td, J = 7.6 and 1.6 Hz, 1H), 7.31 (dd, J = 5.2 and 1.2 Hz, 1H), 7.12 (dd, J = 3.6 and 1.2 Hz, 1H), 6.99 (dd, J = 5.2 and 3.6 Hz, 1H), 3.62 (d, J = 17.2 Hz, 1H), 3.37 (d, J = 17.2 Hz, 1H), 1.99 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 182.3, 177.9, 158.3, 147.3, 134.2, 133.1, 131.7, 127.2, 127.0, 126.4, 126.1, 125.8, 124.2, 123.2, 91.4, 42.2, 29.3; GC/MS (m/z, %): 296.1 (M+, 100). Anal. calcd for C17H12O3S: C, 68.90; H, 4.08; S, 10.82. Found: C, 68.82; H, 4.11; S, 10.89.
O), 1626, 1204 (C–O–C), 955, 722; 1H-NMR (400 MHz, CDCl3) δ 8.11 (dd, J = 6.0 and 1.6 Hz, 1H), 8.08 (dd, J = 6.0 and 1.6 Hz, 1H), 7.73 (td, J = 7.6 and 1.6 Hz, 1H), 7.69 (td, J = 7.6 and 1.6 Hz, 1H), 7.31 (dd, J = 5.2 and 1.2 Hz, 1H), 7.12 (dd, J = 3.6 and 1.2 Hz, 1H), 6.99 (dd, J = 5.2 and 3.6 Hz, 1H), 3.62 (d, J = 17.2 Hz, 1H), 3.37 (d, J = 17.2 Hz, 1H), 1.99 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 182.3, 177.9, 158.3, 147.3, 134.2, 133.1, 131.7, 127.2, 127.0, 126.4, 126.1, 125.8, 124.2, 123.2, 91.4, 42.2, 29.3; GC/MS (m/z, %): 296.1 (M+, 100). Anal. calcd for C17H12O3S: C, 68.90; H, 4.08; S, 10.82. Found: C, 68.82; H, 4.11; S, 10.89.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1657 (C
O), 1657 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1643, 1246 (C–O–C), 984, 728; 1H-NMR (400 MHz, DMSO-D6) δ 7.95 (d, J = 8.0 Hz, 1H), 7.91 (d, J = 1.2 Hz, 1H), 7.50–7.55 (m, 3H), 7.34–7.42 (m, 3H), 7.31 (dd, J = 4.8 and 1.2 Hz, 1H), 6.98 (dd, J = 3.6 and 1.2 Hz, 1H), 6.95 (dd, J = 4.8 and 3.6 Hz, 1H), 4.06 (d, J = 17.6 Hz, 1H), 3.82 (d, J = 17.6 Hz, 1H), 3.19 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 182.4, 178.1, 158.3, 147.6, 144.4, 143.6, 134.9, 131.8, 130.9, 128.8, 128.6, 127.2, 127.1, 127.0, 126.8, 21.9, 126.5, 125.6, 123.5, 93.9, 43.5; LC-MS (ESI, m/z) 373.35 (MH+, 56). Anal. calcd for C23H16O3S: C, 74.17; H, 4.33; S, 8.61. Found: C, 74.06; H, 4.26; S, 8.59.
O), 1643, 1246 (C–O–C), 984, 728; 1H-NMR (400 MHz, DMSO-D6) δ 7.95 (d, J = 8.0 Hz, 1H), 7.91 (d, J = 1.2 Hz, 1H), 7.50–7.55 (m, 3H), 7.34–7.42 (m, 3H), 7.31 (dd, J = 4.8 and 1.2 Hz, 1H), 6.98 (dd, J = 3.6 and 1.2 Hz, 1H), 6.95 (dd, J = 4.8 and 3.6 Hz, 1H), 4.06 (d, J = 17.6 Hz, 1H), 3.82 (d, J = 17.6 Hz, 1H), 3.19 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 182.4, 178.1, 158.3, 147.6, 144.4, 143.6, 134.9, 131.8, 130.9, 128.8, 128.6, 127.2, 127.1, 127.0, 126.8, 21.9, 126.5, 125.6, 123.5, 93.9, 43.5; LC-MS (ESI, m/z) 373.35 (MH+, 56). Anal. calcd for C23H16O3S: C, 74.17; H, 4.33; S, 8.61. Found: C, 74.06; H, 4.26; S, 8.59.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1642 (C
O), 1642 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1597, 1299 (C–O–C), 917, 748; 1H-NMR (400 MHz, CDCl3) δ 8.02 (d, J = 7.6 Hz, 2H) [8.08 (d, J = 7.6 and 1.6 Hz, 2H)], 7.80 (s, 1H) [7.88 (s, 1H)], 7.50 (d, J = 7.6 Hz, 1H) [7.54 (d, J = 7.6 Hz, 1H)], 7.23–7.47 (m, 5H), 6.97 (dd, J = 5.2 and 3.6 Hz, 1H), 6.92 (dd, J = 3.6 and 1.2 Hz, 1H), 6.86 (s, 1H) [6.60 (s, 1H)], 2.45 (s, 3H) [2.48 (s, 3H)]; LC-MS (ESI, m/z) 373.35 (MH+, 18). Anal. calcd for C23H16O3S: C, 74.17; H, 4.33; S, 8.61. Found: C, 74.19; H, 4.32; S, 8.59.
O), 1597, 1299 (C–O–C), 917, 748; 1H-NMR (400 MHz, CDCl3) δ 8.02 (d, J = 7.6 Hz, 2H) [8.08 (d, J = 7.6 and 1.6 Hz, 2H)], 7.80 (s, 1H) [7.88 (s, 1H)], 7.50 (d, J = 7.6 Hz, 1H) [7.54 (d, J = 7.6 Hz, 1H)], 7.23–7.47 (m, 5H), 6.97 (dd, J = 5.2 and 3.6 Hz, 1H), 6.92 (dd, J = 3.6 and 1.2 Hz, 1H), 6.86 (s, 1H) [6.60 (s, 1H)], 2.45 (s, 3H) [2.48 (s, 3H)]; LC-MS (ESI, m/z) 373.35 (MH+, 18). Anal. calcd for C23H16O3S: C, 74.17; H, 4.33; S, 8.61. Found: C, 74.19; H, 4.32; S, 8.59.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1646 (C
O), 1646 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1601, 1232 (C–O–C), 1054, 717; 1H-NMR (400 MHz, CDCl3) δ 7.85 (s, 1H), 7.54 (dd, J = 8.0 and 1.6 Hz, 2H), 7.33–7.40 (m, 3H), 7.30 (dd, J = 5.2 and 1.2 Hz, 1H), 7.28 (s, 1H), 6.97 (dd, J = 3.6 and 1.2 Hz, 1H), 6.93 (dd, J = 5.2 and 3.6 Hz, 1H), 4.02 (d, J = 17.6 Hz, 1H), 3.79 (d, J = 17.6 Hz, 1H), 2.70 (s, 3H), 2.41 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 184.8, 178.2, 156.8, 147.7, 143.7, 143.1, 141.4, 139.3, 133.5, 128.7, 128.6, 128.0, 127.1, 126.9, 126.7, 126.4, 125.7, 124.9, 93.6, 43.9, 22.9, 21.6; LC/MS, (ESI, m/z): 387.48 (MH+, 100). Anal. calcd for C24H18O3S: C, 74.59; H, 4.69; S, 8.30. Found: C, 74.53; H, 4.65; S, 8.33.
O), 1601, 1232 (C–O–C), 1054, 717; 1H-NMR (400 MHz, CDCl3) δ 7.85 (s, 1H), 7.54 (dd, J = 8.0 and 1.6 Hz, 2H), 7.33–7.40 (m, 3H), 7.30 (dd, J = 5.2 and 1.2 Hz, 1H), 7.28 (s, 1H), 6.97 (dd, J = 3.6 and 1.2 Hz, 1H), 6.93 (dd, J = 5.2 and 3.6 Hz, 1H), 4.02 (d, J = 17.6 Hz, 1H), 3.79 (d, J = 17.6 Hz, 1H), 2.70 (s, 3H), 2.41 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 184.8, 178.2, 156.8, 147.7, 143.7, 143.1, 141.4, 139.3, 133.5, 128.7, 128.6, 128.0, 127.1, 126.9, 126.7, 126.4, 125.7, 124.9, 93.6, 43.9, 22.9, 21.6; LC/MS, (ESI, m/z): 387.48 (MH+, 100). Anal. calcd for C24H18O3S: C, 74.59; H, 4.69; S, 8.30. Found: C, 74.53; H, 4.65; S, 8.33.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1654 (C
O), 1654 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1630; 1H-NMR (400 MHz, CDCl3) δ 7.75 (s, 1H) [7.82 (s, 1H)], [7.48 (m, 2H)], 7.23–7.36 (m, 6H), 7.11 (s, 1H), 6.98 (dd, J = 5.2 and 3.6 Hz, 1H), 6.90–6.93 (m, 1H), 6.90 (s, 1H) [6.64 (s, 1H)], [2.42 (s, 3H), 2.45 (s, 3H)], 2.39 (s, 3H), 2.38 (s, 3H); LC/MS, (ESI, m/z): 387.48 (MH+, 60). Anal. calcd for C24H18O3S: C, 74.59; H, 4.69; S, 8.30. Found: C, 74.60; H, 4.75; S, 8.32.
O), 1630; 1H-NMR (400 MHz, CDCl3) δ 7.75 (s, 1H) [7.82 (s, 1H)], [7.48 (m, 2H)], 7.23–7.36 (m, 6H), 7.11 (s, 1H), 6.98 (dd, J = 5.2 and 3.6 Hz, 1H), 6.90–6.93 (m, 1H), 6.90 (s, 1H) [6.64 (s, 1H)], [2.42 (s, 3H), 2.45 (s, 3H)], 2.39 (s, 3H), 2.38 (s, 3H); LC/MS, (ESI, m/z): 387.48 (MH+, 60). Anal. calcd for C24H18O3S: C, 74.59; H, 4.69; S, 8.30. Found: C, 74.60; H, 4.75; S, 8.32.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1656 (C
O), 1656 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1584, 1247 (C–O–C), 965, 698; 1H-NMR (400 MHz, CDCl3) δ 8.05 (d, J = 8.4 Hz, 1H), 7.53–7.55 (m, 3H), 7.34–7.41 (m, 3H), 7.32 (dd, J = 4.8 and 1.2 Hz, 1H), 7.12 (d, J = 8.4 ve 2.8 Hz, 1H), 6.98 (dd, J = 3.6 and 1.2 Hz, 1H), 6.95 (dd, J = 4.8 and 3.6 Hz, 1H), 4.05 (d, J = 17.6 Hz, 1H), 3.94 (s, 3H), 3.82 (d, J = 17.6 Hz, 1H); 13C-NMR (100 MHz, CDCl3) δ 182.2, 176.9, 164.8, 158.9, 147.5, 143.6, 135.7, 129.2, 128.8, 128.6, 127.1, 127.0, 126.9, 125.6, 125.2, 122.9, 118.8, 110.9, 94.1, 56.2, 43.5; GC-MS (m/z, %) 388.1 (M+, 100). Anal. calcd for C23H16O4S: C, 71.12; H, 4.15; S, 8.25. Found: C, 71.34; H, 4.07 S, 8.31.
O), 1584, 1247 (C–O–C), 965, 698; 1H-NMR (400 MHz, CDCl3) δ 8.05 (d, J = 8.4 Hz, 1H), 7.53–7.55 (m, 3H), 7.34–7.41 (m, 3H), 7.32 (dd, J = 4.8 and 1.2 Hz, 1H), 7.12 (d, J = 8.4 ve 2.8 Hz, 1H), 6.98 (dd, J = 3.6 and 1.2 Hz, 1H), 6.95 (dd, J = 4.8 and 3.6 Hz, 1H), 4.05 (d, J = 17.6 Hz, 1H), 3.94 (s, 3H), 3.82 (d, J = 17.6 Hz, 1H); 13C-NMR (100 MHz, CDCl3) δ 182.2, 176.9, 164.8, 158.9, 147.5, 143.6, 135.7, 129.2, 128.8, 128.6, 127.1, 127.0, 126.9, 125.6, 125.2, 122.9, 118.8, 110.9, 94.1, 56.2, 43.5; GC-MS (m/z, %) 388.1 (M+, 100). Anal. calcd for C23H16O4S: C, 71.12; H, 4.15; S, 8.25. Found: C, 71.34; H, 4.07 S, 8.31.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1588, 1309, 1248 (C–O–C), 700; 1H-NMR (400 MHz, CDCl3) δ 7.94 (d, J = 8.8 Hz, 1H) [8.01 (d, J = 8.4 Hz, 1H)], 7.58 (s, 1H), 7.46 (d, J = 2.8 Hz, 1H) [7.51 (d, J = 2.8 Hz, 1H)], 7.23–7.37 (m, 5H), 7.08 (dd, J = 8.8 and 2.8 Hz, 1H) [7.12 (dd, J = 8.4 and 2.4 Hz, 1H)], 6.97 (dd, J = 5.2 and 4.0 Hz, 1H), 6.93 (m, 1H), 6.90 (dd, J = 3.6 and 1.2 Hz, 1H), 6.84 (s, 1H) [6.57 (s, 1H)], 3.92 (s, 3H) [3.93 (s, 3H)]; GC-MS (m/z, %) 388 (M+, 100). Anal. calcd for C23H16O4S: C, 71.12; H, 4.15; S, 8.25. Found: C, 71.09; H, 4.07; S, 8.28.
O), 1588, 1309, 1248 (C–O–C), 700; 1H-NMR (400 MHz, CDCl3) δ 7.94 (d, J = 8.8 Hz, 1H) [8.01 (d, J = 8.4 Hz, 1H)], 7.58 (s, 1H), 7.46 (d, J = 2.8 Hz, 1H) [7.51 (d, J = 2.8 Hz, 1H)], 7.23–7.37 (m, 5H), 7.08 (dd, J = 8.8 and 2.8 Hz, 1H) [7.12 (dd, J = 8.4 and 2.4 Hz, 1H)], 6.97 (dd, J = 5.2 and 4.0 Hz, 1H), 6.93 (m, 1H), 6.90 (dd, J = 3.6 and 1.2 Hz, 1H), 6.84 (s, 1H) [6.57 (s, 1H)], 3.92 (s, 3H) [3.93 (s, 3H)]; GC-MS (m/z, %) 388 (M+, 100). Anal. calcd for C23H16O4S: C, 71.12; H, 4.15; S, 8.25. Found: C, 71.09; H, 4.07; S, 8.28.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1655 (C
O), 1655 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1643, 1240 (C–O–C), 984, 714; 1H-NMR (400 MHz, CDCl3) δ 7.95 (d, J = 8.0 Hz, 1H), 7.90 (s, 1H), 7.50 (d, J = 8.0 Hz, 1H), 7.41 (d, J = 8.0 Hz, 2H), 7.30 (d, J = 4.8, 1H), 7.19 (d, J = 8.0 Hz, 2H), 6.96–6.94 (m, 2H), 4.02 (d, J = 17.6 Hz, 1H), 3.80 (d, J = 17.6 Hz, 1H), 2.47 (s, 3H), 2.36 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 182.5, 178.2, 158.3, 147.8, 144.3, 140.6, 138.5, 134.9, 131.8, 130.9, 129.4, 127.2, 127.0, 126.7, 126.5, 125.6, 123.6, 94.0, 43.4, 21.9, 21.3; LC-MS (ESI, m/z): 387.39 (M+ + H, 15), 409.34 (M + Na+, 100), 450.36 (M + Na+ + CH3CN, 51). Anal. calcd for C24H18O3S: C, 74.59; H, 4.69; S, 8.30. Found: C, 74.64; H, 4.62; S, 8.32.
O), 1643, 1240 (C–O–C), 984, 714; 1H-NMR (400 MHz, CDCl3) δ 7.95 (d, J = 8.0 Hz, 1H), 7.90 (s, 1H), 7.50 (d, J = 8.0 Hz, 1H), 7.41 (d, J = 8.0 Hz, 2H), 7.30 (d, J = 4.8, 1H), 7.19 (d, J = 8.0 Hz, 2H), 6.96–6.94 (m, 2H), 4.02 (d, J = 17.6 Hz, 1H), 3.80 (d, J = 17.6 Hz, 1H), 2.47 (s, 3H), 2.36 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 182.5, 178.2, 158.3, 147.8, 144.3, 140.6, 138.5, 134.9, 131.8, 130.9, 129.4, 127.2, 127.0, 126.7, 126.5, 125.6, 123.6, 94.0, 43.4, 21.9, 21.3; LC-MS (ESI, m/z): 387.39 (M+ + H, 15), 409.34 (M + Na+, 100), 450.36 (M + Na+ + CH3CN, 51). Anal. calcd for C24H18O3S: C, 74.59; H, 4.69; S, 8.30. Found: C, 74.64; H, 4.62; S, 8.32.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1599, 1352 (C–O–C), 739; 1H-NMR (400 MHz, CDCl3) δ [7.99 (d, J = 8.0 Hz, 1H)] 7.96 (d, J = 8.0 Hz, 1H), 7.95 (s, 1H), 7.79 (d, J = 8.4 Hz, 2H), 7.56 (d, J = 8.0 Hz, 1H) [7.52 (d, J = 8.0 Hz, 1H)], [7.50 (dd, J = 4.0 and 1.2 Hz, 1H)], 7.32 (dd, J = 5.2 and 1.2 Hz, 1H), 7.25 (d, J = 8.4 Hz, 2H), [7.21 (d, J = 8.4 Hz, 2H), 7.12 (d, J = 8.4 Hz, 2H)], 6.92 (dd, J = 5.2 and 4.0 Hz, 1H) [7.05 (dd, J = 5.2 and 4.0 Hz, 1H)], 6.90 (s, 1H) [7.01 (s, 1H)], 6.82 (dd, J = 3.2 and 1.2 Hz, 1H), 2.49 (s, 3H) [2.34 (s, 3H), 2.37 (s, 3H)], 1.84 (s, 3H); LC-MS (ESI, m/z): 387.56 (M+ + H, 13), 409.53 (M + Na+, 100), 450.42 (M + Na+ + CH3CN, 50). Anal. calcd for C24H18O3S: C, 74.59; H, 4.69; S, 8.30. Found: C, 74.63; H, 4.71; S, 8.31.
O), 1599, 1352 (C–O–C), 739; 1H-NMR (400 MHz, CDCl3) δ [7.99 (d, J = 8.0 Hz, 1H)] 7.96 (d, J = 8.0 Hz, 1H), 7.95 (s, 1H), 7.79 (d, J = 8.4 Hz, 2H), 7.56 (d, J = 8.0 Hz, 1H) [7.52 (d, J = 8.0 Hz, 1H)], [7.50 (dd, J = 4.0 and 1.2 Hz, 1H)], 7.32 (dd, J = 5.2 and 1.2 Hz, 1H), 7.25 (d, J = 8.4 Hz, 2H), [7.21 (d, J = 8.4 Hz, 2H), 7.12 (d, J = 8.4 Hz, 2H)], 6.92 (dd, J = 5.2 and 4.0 Hz, 1H) [7.05 (dd, J = 5.2 and 4.0 Hz, 1H)], 6.90 (s, 1H) [7.01 (s, 1H)], 6.82 (dd, J = 3.2 and 1.2 Hz, 1H), 2.49 (s, 3H) [2.34 (s, 3H), 2.37 (s, 3H)], 1.84 (s, 3H); LC-MS (ESI, m/z): 387.56 (M+ + H, 13), 409.53 (M + Na+, 100), 450.42 (M + Na+ + CH3CN, 50). Anal. calcd for C24H18O3S: C, 74.59; H, 4.69; S, 8.30. Found: C, 74.63; H, 4.71; S, 8.31.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1644 (C
O), 1644 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1636, 1270 (C–O–C), 1052, 982, 714; 1H-NMR (400 MHz, CDCl3) δ 7.84 (s, 1H), 7.41 (d, J = 8.0 Hz, 2H), 7.29 (d, J = 5.2 Hz, 1H), 7.27 (s, 1H), 7.18 (d, J = 8.0 Hz, 2H), 6.92–6.97 (m, 2H), 4.00 (d, J = 17.6 Hz, 1H), 3.77 (d, J = 17.6 Hz, 1H), 2.69 (s, 3H), 2.40 (s, 3H), 2.35 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 184.9, 178.3, 156.9, 148.0, 143.1, 141.4, 140.8, 139.3, 138.4, 133.5, 129.4, 128.0, 127.0, 126.9, 126.6, 126.4, 125.6, 125.0, 93.7, 43.8, 22.9, 21.6, 21.4; LC/MS, (ESI, m/z): 401.99 (M + H+, 12), 423.90 (M + Na+, 15), 464 (M + Na+ + CH3CN, 100). Anal. calcd for C25H20O3S: C, 74.98; H, 5.03; S, 8.01. Found: C, 74.93; H, 5.06; S, 7.99.
O), 1636, 1270 (C–O–C), 1052, 982, 714; 1H-NMR (400 MHz, CDCl3) δ 7.84 (s, 1H), 7.41 (d, J = 8.0 Hz, 2H), 7.29 (d, J = 5.2 Hz, 1H), 7.27 (s, 1H), 7.18 (d, J = 8.0 Hz, 2H), 6.92–6.97 (m, 2H), 4.00 (d, J = 17.6 Hz, 1H), 3.77 (d, J = 17.6 Hz, 1H), 2.69 (s, 3H), 2.40 (s, 3H), 2.35 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 184.9, 178.3, 156.9, 148.0, 143.1, 141.4, 140.8, 139.3, 138.4, 133.5, 129.4, 128.0, 127.0, 126.9, 126.6, 126.4, 125.6, 125.0, 93.7, 43.8, 22.9, 21.6, 21.4; LC/MS, (ESI, m/z): 401.99 (M + H+, 12), 423.90 (M + Na+, 15), 464 (M + Na+ + CH3CN, 100). Anal. calcd for C25H20O3S: C, 74.98; H, 5.03; S, 8.01. Found: C, 74.93; H, 5.06; S, 7.99.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1598, 1293 (C–O–C), 1089, 727; 1H-NMR (400 MHz, CDCl3) δ 7.75 (s, 1H) [7.81 (s, 1H)], [7.37 (d, J = 7.6 Hz, 2H)], 7.25–7.30 (m, 2H + [2H]), 7.19 (d, J = 7.6 Hz, 2H), 7.04 (d, J = 7.6 Hz, 2H) [7.15 (d, J = 8.0 Hz, 2H)], 6.91–6.98 (m, 2H + [2H]), 6.86 (s, 1H) [6.62 (s, 1H)], 2.39 (s, 3H) [2.41 (s, 3H), 2.43 (s, 3H)], 2.37 (s, 3H + [3H]), 2.31 (s, 3H); LC/MS, (ESI, m/z): 401.53 (M + H+, 50), 423.41 (M + Na+, 30), 464.53 (M + Na+ + CH3CN, 100). Anal. calcd for C25H20O3S: C, 74.98; H, 5.03; S, 8.01. Found: C, 74.87; H, 5.02; S, 8.01.
O), 1598, 1293 (C–O–C), 1089, 727; 1H-NMR (400 MHz, CDCl3) δ 7.75 (s, 1H) [7.81 (s, 1H)], [7.37 (d, J = 7.6 Hz, 2H)], 7.25–7.30 (m, 2H + [2H]), 7.19 (d, J = 7.6 Hz, 2H), 7.04 (d, J = 7.6 Hz, 2H) [7.15 (d, J = 8.0 Hz, 2H)], 6.91–6.98 (m, 2H + [2H]), 6.86 (s, 1H) [6.62 (s, 1H)], 2.39 (s, 3H) [2.41 (s, 3H), 2.43 (s, 3H)], 2.37 (s, 3H + [3H]), 2.31 (s, 3H); LC/MS, (ESI, m/z): 401.53 (M + H+, 50), 423.41 (M + Na+, 30), 464.53 (M + Na+ + CH3CN, 100). Anal. calcd for C25H20O3S: C, 74.98; H, 5.03; S, 8.01. Found: C, 74.87; H, 5.02; S, 8.01.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1590, 1246 (C–O–C), 967, 699; 1H-NMR (400 MHz, CDCl3) δ 8.04 (d, J = 8.8 Hz, 1H), 7.52 (d, J = 2.8 Hz, 1H), 7.41 (d, J = 8.0 Hz, 2H), 7.30 (dd, J = 5.2 and 1.2 Hz, 1H), 7.19 (d, J = 8.0 Hz, 2H), 7.10 (d, J = 8.8 and 2.8 Hz, 1H), 6.97 (dd, J = 3.6 and 1.2 Hz, 1H), 6.94 (dd, J = 5.2 and 3.6 Hz, 1H), 4.02 (d, J = 17.6 Hz, 1H), 3.93 (s, 3H), 3.80 (d, J = 17.6 Hz, 1H), 2.35 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 182.2, 176.9, 164.8, 158.9, 147.7, 140.6, 138.5, 135.6, 129.4, 129.2, 127.0, 126.7, 125.6, 125.2, 123.0, 118.8, 110.8, 94.2, 56.2, 43.4, 21.3; LC-MS (ESI, m/z) 403.84 (M + H+, 100). Anal. calcd for C24H18O4S: C, 71.62; H, 4.51; S, 7.97. Found: C, 71.76; H, 4.53; S, 7.94.
O), 1590, 1246 (C–O–C), 967, 699; 1H-NMR (400 MHz, CDCl3) δ 8.04 (d, J = 8.8 Hz, 1H), 7.52 (d, J = 2.8 Hz, 1H), 7.41 (d, J = 8.0 Hz, 2H), 7.30 (dd, J = 5.2 and 1.2 Hz, 1H), 7.19 (d, J = 8.0 Hz, 2H), 7.10 (d, J = 8.8 and 2.8 Hz, 1H), 6.97 (dd, J = 3.6 and 1.2 Hz, 1H), 6.94 (dd, J = 5.2 and 3.6 Hz, 1H), 4.02 (d, J = 17.6 Hz, 1H), 3.93 (s, 3H), 3.80 (d, J = 17.6 Hz, 1H), 2.35 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 182.2, 176.9, 164.8, 158.9, 147.7, 140.6, 138.5, 135.6, 129.4, 129.2, 127.0, 126.7, 125.6, 125.2, 123.0, 118.8, 110.8, 94.2, 56.2, 43.4, 21.3; LC-MS (ESI, m/z) 403.84 (M + H+, 100). Anal. calcd for C24H18O4S: C, 71.62; H, 4.51; S, 7.97. Found: C, 71.76; H, 4.53; S, 7.94.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1589, 1248 (C–O–C), 819; 1H-NMR (400 MHz, CDCl3) δ 7.96 (d, J = 8.8 Hz, 1H) [8.02 (d, J = 8.4 Hz, 1H)], 7.48 (d, J = 2.4 Hz, 1H) [7.51 (d, J = 2.4 Hz, 1H)], [7.35 (d, J = 8.0 Hz, 2H)], 7.32 (s, 1H, OH) [7.15 (s, 1H, OH)], [7.28 (dd, J = 4.8 and 1.2 Hz, 1H), 7.24 (dd, J = 4.8 and 2.0 Hz, 1H)], 7.18 (d, J = 8.0 Hz, 2H), 7.09 (dd, J = 8.8 and 2.8 Hz, 1H) [7.13 (dd, J = 8.8 and 2.8 Hz, 1H)], 7.06 (d, J = 8.4 Hz, 2H), 6.98 (dd, J = 5.2 and 3.6 Hz, 1H), 6.92–6.94 (m, 2H), 6.80 (s, 1H) [6.55 (s, 1H)], 3.93 (s, 3H) [3.94 (s, 3H)], 2.32 (s, 3H) [2.39 (s, 3H)]; LC-MS (ES+, m/z) 403.53 (M + H+, 100). Anal. calcd for C24H18O4S: C, 71.62; H, 4.51; S, 7.97. Found: C, 71.61; H, 4.49; S, 7.96.
O), 1589, 1248 (C–O–C), 819; 1H-NMR (400 MHz, CDCl3) δ 7.96 (d, J = 8.8 Hz, 1H) [8.02 (d, J = 8.4 Hz, 1H)], 7.48 (d, J = 2.4 Hz, 1H) [7.51 (d, J = 2.4 Hz, 1H)], [7.35 (d, J = 8.0 Hz, 2H)], 7.32 (s, 1H, OH) [7.15 (s, 1H, OH)], [7.28 (dd, J = 4.8 and 1.2 Hz, 1H), 7.24 (dd, J = 4.8 and 2.0 Hz, 1H)], 7.18 (d, J = 8.0 Hz, 2H), 7.09 (dd, J = 8.8 and 2.8 Hz, 1H) [7.13 (dd, J = 8.8 and 2.8 Hz, 1H)], 7.06 (d, J = 8.4 Hz, 2H), 6.98 (dd, J = 5.2 and 3.6 Hz, 1H), 6.92–6.94 (m, 2H), 6.80 (s, 1H) [6.55 (s, 1H)], 3.93 (s, 3H) [3.94 (s, 3H)], 2.32 (s, 3H) [2.39 (s, 3H)]; LC-MS (ES+, m/z) 403.53 (M + H+, 100). Anal. calcd for C24H18O4S: C, 71.62; H, 4.51; S, 7.97. Found: C, 71.61; H, 4.49; S, 7.96.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1656 (C
O), 1656 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1642, 1240 (C–O–C), 984, 712; 1H-NMR (400 MHz, CDCl3) δ 7.95 (d, J = 8.0 Hz, 1H), 7.90 (s, 1H), 7.50 (m, 3H), 7.32 (dd, J = 4.8 and 1.6, 1H), 7.08 (t, J = 8.8 Hz, 2H), 6.94–6.98 (m, 2H), 4.04 (d, J = 17.2 Hz, 1H), 3.78 (d, J = 17.2 Hz, 1H), 2.48 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 182.3, 178.0, 164.0, 161.5, 158.1, 147.3, 144.5, 139.4 (Cpara, i, J = 3.2 Hz), 135.0, 131.8, 130.9, 127.6 (Cmeta, i, J = 8.4 Hz), 127.3, 127.2, 127.1, 126.8, 126.5, 123.4, 115.7 (Cortho, i, J = 21.8 Hz), 93.5, 43.5, 21.9; LC/MS, (ESI, m/z): 391.34 (M+ + H, 100), 413.32 (M + Na+, 37), 454.33 (M + Na+ + CH3CN, 100). Anal. calcd for C23H15FO3S: C, 70.75; H, 3.87; S, 8.21. Found: C, 70.80; H, 3.91 S, 8.22.
O), 1642, 1240 (C–O–C), 984, 712; 1H-NMR (400 MHz, CDCl3) δ 7.95 (d, J = 8.0 Hz, 1H), 7.90 (s, 1H), 7.50 (m, 3H), 7.32 (dd, J = 4.8 and 1.6, 1H), 7.08 (t, J = 8.8 Hz, 2H), 6.94–6.98 (m, 2H), 4.04 (d, J = 17.2 Hz, 1H), 3.78 (d, J = 17.2 Hz, 1H), 2.48 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 182.3, 178.0, 164.0, 161.5, 158.1, 147.3, 144.5, 139.4 (Cpara, i, J = 3.2 Hz), 135.0, 131.8, 130.9, 127.6 (Cmeta, i, J = 8.4 Hz), 127.3, 127.2, 127.1, 126.8, 126.5, 123.4, 115.7 (Cortho, i, J = 21.8 Hz), 93.5, 43.5, 21.9; LC/MS, (ESI, m/z): 391.34 (M+ + H, 100), 413.32 (M + Na+, 37), 454.33 (M + Na+ + CH3CN, 100). Anal. calcd for C23H15FO3S: C, 70.75; H, 3.87; S, 8.21. Found: C, 70.80; H, 3.91 S, 8.22.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1598, 1298 (C–O–C), 851, 717; 1H-NMR (400 MHz, CDCl3) δ 7.92 (d, J = 8.0 Hz, 1H) [7.95 (d, J = 8.0 Hz, 1H)], 7.82 (d, J = 0.8 Hz, 1H) [7.88 (d, J = 0.8 Hz, 1H)], 7.52 (dd, J = 7.6 and 0.8 Hz, 1H) [7.55 (dd, J = 7.6 and 0.8 Hz, 1H)], [7.44 (m, 2H), 7.40 (s, 1H)], 7.23–7.31 (m, 4H + [3H]) 6.98 (dd, J = 5.2 and 3.6 Hz, 1H), 6.94 (td, J = 8.0 and 1.6 Hz, 2H) [7.05 (t, J = 8.8 Hz, 2H)], 6.89 (dd, J = 3.6 and 1.2 Hz, 1H), 6.85 (s, 1H) [6.55 (s, 1H)], 2.46 (s, 3H) [2.48 (s, 3H)]; LC/MS, (ESI, m/z): 391.35 (M+ + H, 60), 413.30 (M + Na+, 20), 454.34 (M + Na+ + CH3CN, 10). Anal. calcd for C23H15FO3S: C, 70.75; H, 3.87; S, 8.21. Found: C, 70.82; H, 3.94; S, 8.21.
O), 1598, 1298 (C–O–C), 851, 717; 1H-NMR (400 MHz, CDCl3) δ 7.92 (d, J = 8.0 Hz, 1H) [7.95 (d, J = 8.0 Hz, 1H)], 7.82 (d, J = 0.8 Hz, 1H) [7.88 (d, J = 0.8 Hz, 1H)], 7.52 (dd, J = 7.6 and 0.8 Hz, 1H) [7.55 (dd, J = 7.6 and 0.8 Hz, 1H)], [7.44 (m, 2H), 7.40 (s, 1H)], 7.23–7.31 (m, 4H + [3H]) 6.98 (dd, J = 5.2 and 3.6 Hz, 1H), 6.94 (td, J = 8.0 and 1.6 Hz, 2H) [7.05 (t, J = 8.8 Hz, 2H)], 6.89 (dd, J = 3.6 and 1.2 Hz, 1H), 6.85 (s, 1H) [6.55 (s, 1H)], 2.46 (s, 3H) [2.48 (s, 3H)]; LC/MS, (ESI, m/z): 391.35 (M+ + H, 60), 413.30 (M + Na+, 20), 454.34 (M + Na+ + CH3CN, 10). Anal. calcd for C23H15FO3S: C, 70.75; H, 3.87; S, 8.21. Found: C, 70.82; H, 3.94; S, 8.21.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1648 (C
O), 1648 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1600, 1223 (C–O–C), 836, 716; 1H-NMR (400 MHz, CDCl3) δ 7.85 (d, J = 1.6 Hz, 1H), 7.51 (m, 2H), 7.32 (dd, J = 4.4 and 1.6 Hz, 1H), 7.29 (d, J = 1.6 Hz, 1H), 7.07 (td, J = 8.8 and 1.0 Hz, 2H), 6.94–6.98 (m, 2H), 4.02 (d, J = 17.6 Hz, 1H), 3.75 (d, J = 17.6 Hz, 1H), 2.70 (s, 3H), 2.42 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 184.8, 178.2, 164.0, 161.5, 156.7, 147.5, 143.3, 141.5, 139.5 (Cpara, i, J = 3.2 Hz), 139.4, 133.5, 128.0, 127.7 (Cmeta, i, J = 8.3 Hz), 127.2, 127.1, 126.8, 126.4, 124.9, 115.7 (Cortho, i, J = 21.2 Hz), 93.2, 44.0, 23.0, 21.6; LC/MS, (ESI, m/z): 405.34 (M + H+, 100), 427.31 (M + Na+, 20), 468.32 (M + Na+ + CH3CN, 95). Anal. calcd for C24H17FO3S: C, 71.27; H, 4.24; S, 7.93. Found: C, 71.34; H, 4.20; S, 7.90.
O), 1600, 1223 (C–O–C), 836, 716; 1H-NMR (400 MHz, CDCl3) δ 7.85 (d, J = 1.6 Hz, 1H), 7.51 (m, 2H), 7.32 (dd, J = 4.4 and 1.6 Hz, 1H), 7.29 (d, J = 1.6 Hz, 1H), 7.07 (td, J = 8.8 and 1.0 Hz, 2H), 6.94–6.98 (m, 2H), 4.02 (d, J = 17.6 Hz, 1H), 3.75 (d, J = 17.6 Hz, 1H), 2.70 (s, 3H), 2.42 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 184.8, 178.2, 164.0, 161.5, 156.7, 147.5, 143.3, 141.5, 139.5 (Cpara, i, J = 3.2 Hz), 139.4, 133.5, 128.0, 127.7 (Cmeta, i, J = 8.3 Hz), 127.2, 127.1, 126.8, 126.4, 124.9, 115.7 (Cortho, i, J = 21.2 Hz), 93.2, 44.0, 23.0, 21.6; LC/MS, (ESI, m/z): 405.34 (M + H+, 100), 427.31 (M + Na+, 20), 468.32 (M + Na+ + CH3CN, 95). Anal. calcd for C24H17FO3S: C, 71.27; H, 4.24; S, 7.93. Found: C, 71.34; H, 4.20; S, 7.90.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1597, 1337, 1091 (C–O–C), 705; 1H-NMR (400 MHz, CDCl3) δ 7.76 (d, J = 1.6 Hz, 1H) [7.82 (d, J = 1.2 Hz, 1H)], [7.45 (dd, J = 8.8 and 5.2 Hz, 2H)], 7.26–7.32 (m, 4H + [4H]), 7.14 (s, 1H), 6.98 (dd, J = 5.2 and 3.6 Hz, 1H), 6.94 (td, J = 8.8 and 2.4 Hz, 1H) [7.04 (td, J = 8.8 and 2.4 Hz, 2H)], 6.90–6.93 (m, 1H), 6.88 (s, 1H) [6.59 (s, 1H)], 2.45 (s, 3H) [2.42 (s, 3H), 2.45 (s, 3H)], 2.40 (s, 3H); LC/MS, (ESI, m/z): 405.45 (M + H+, 100). Anal. calcd for C24H17FO3S: C, 71.27; H, 4.24; S, 7.93. Found: C, 71.33; H, 4.32; S, 7.96.
O), 1597, 1337, 1091 (C–O–C), 705; 1H-NMR (400 MHz, CDCl3) δ 7.76 (d, J = 1.6 Hz, 1H) [7.82 (d, J = 1.2 Hz, 1H)], [7.45 (dd, J = 8.8 and 5.2 Hz, 2H)], 7.26–7.32 (m, 4H + [4H]), 7.14 (s, 1H), 6.98 (dd, J = 5.2 and 3.6 Hz, 1H), 6.94 (td, J = 8.8 and 2.4 Hz, 1H) [7.04 (td, J = 8.8 and 2.4 Hz, 2H)], 6.90–6.93 (m, 1H), 6.88 (s, 1H) [6.59 (s, 1H)], 2.45 (s, 3H) [2.42 (s, 3H), 2.45 (s, 3H)], 2.40 (s, 3H); LC/MS, (ESI, m/z): 405.45 (M + H+, 100). Anal. calcd for C24H17FO3S: C, 71.27; H, 4.24; S, 7.93. Found: C, 71.33; H, 4.32; S, 7.96.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1654 (C
O), 1654 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1583, 1246 (C–O–C), 948, 714; 1H-NMR (400 MHz, CDCl3) δ 8.04 (d, J = 8.8 Hz, 1H), 7.53 (d, J = 2.4 Hz, 1H), 7.50 (ddd, J = 8.4 and 5.2 and 2.0 Hz, 2H), 7.32 (dd, J = 5.2 and 1.6 Hz, 1H), 7.11 (dd, J = 8.8 and 2.4 Hz, 1H), 7.07 (td, J = 8.4 and 2.0 Hz, 2H), 6.94–6.98 (s, 2H), 4.04 (d, J = 17.2 Hz, 1H), 3.94 (s, 3H), 3.77 (d, J = 17.2 Hz, 1H); 13C-NMR (100 MHz, CDCl3) δ 182.1, 176.8, 164.8, 162.5 (Cipso, i, J = 246.1 Hz), 158.8, 147.3, 139.4 (Cpara, i, J = 3.11 Hz), 135.6, 129.2, 127.6 (Cmeta, i, J = 8.4 Hz), 127.2, 127.1, 126.8, 125.2, 122.8, 118.8, 115.7 (Cortho, i, J = 21.3 Hz), 110.9, 93.7, 56.2, 43.5; LC-MS (ESI, m/z) 407.46 (M + H+, 98), 429.57 (M + Na+, 35), 470.22 (M + Na+ + CH3CN, 100). Anal. calcd for C23H15FO4S: C, 67.97; H, 3.72; S, 7.89. Found: C, 68.04; H, 3.64; S, 7.93.
O), 1583, 1246 (C–O–C), 948, 714; 1H-NMR (400 MHz, CDCl3) δ 8.04 (d, J = 8.8 Hz, 1H), 7.53 (d, J = 2.4 Hz, 1H), 7.50 (ddd, J = 8.4 and 5.2 and 2.0 Hz, 2H), 7.32 (dd, J = 5.2 and 1.6 Hz, 1H), 7.11 (dd, J = 8.8 and 2.4 Hz, 1H), 7.07 (td, J = 8.4 and 2.0 Hz, 2H), 6.94–6.98 (s, 2H), 4.04 (d, J = 17.2 Hz, 1H), 3.94 (s, 3H), 3.77 (d, J = 17.2 Hz, 1H); 13C-NMR (100 MHz, CDCl3) δ 182.1, 176.8, 164.8, 162.5 (Cipso, i, J = 246.1 Hz), 158.8, 147.3, 139.4 (Cpara, i, J = 3.11 Hz), 135.6, 129.2, 127.6 (Cmeta, i, J = 8.4 Hz), 127.2, 127.1, 126.8, 125.2, 122.8, 118.8, 115.7 (Cortho, i, J = 21.3 Hz), 110.9, 93.7, 56.2, 43.5; LC-MS (ESI, m/z) 407.46 (M + H+, 98), 429.57 (M + Na+, 35), 470.22 (M + Na+ + CH3CN, 100). Anal. calcd for C23H15FO4S: C, 67.97; H, 3.72; S, 7.89. Found: C, 68.04; H, 3.64; S, 7.93.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1589, 1248 (C–O–C), 973, 701; 1H-NMR (400 MHz, CDCl3) δ 7.97 (d, J = 8.8 Hz, 1H) [8.02 (d, J = 8.0 Hz, 1H)], 7.47 (d, J = 2.8 Hz, 1H) [7.51 (d, J = 2.4 Hz, 1H)], 7.38 (s, 1H, OH) [7.57 (s, 1H, OH)], [7.42 (ddd, J = 8.8 and 6.4 and 2.4 Hz, 2H)], 7.28–7.30 (m, 3H), 7.11 (dd, J = 8.4 and 2.8 Hz, 7H) [7.12 (dd, J = 8.8 and 2.8 Hz, 1H)], 7.00 (dd, J = 4.8 and 3.6 Hz, 1H), 6.95 (td, J = 8.4 and 1.0 Hz, 2H) [7.06 (td, J = 8.4 and 1.0 Hz, 2H)], 6.89 (dd, J = 3.6 and 1.2 Hz, 1H), 6.82 (s, 1H) [6.52 (s, 1H)], 3.93 (s, 3H) [3.94 (s, 3H)]; LC-MS (ESI, m/z) 407.37 (M + H+, 100). Anal. calcd for C23H15FO4S: C, 67.97; H, 3.72; S, 7.89. Found: C, 67.93; H, 3.78; S, 7.87.
O), 1589, 1248 (C–O–C), 973, 701; 1H-NMR (400 MHz, CDCl3) δ 7.97 (d, J = 8.8 Hz, 1H) [8.02 (d, J = 8.0 Hz, 1H)], 7.47 (d, J = 2.8 Hz, 1H) [7.51 (d, J = 2.4 Hz, 1H)], 7.38 (s, 1H, OH) [7.57 (s, 1H, OH)], [7.42 (ddd, J = 8.8 and 6.4 and 2.4 Hz, 2H)], 7.28–7.30 (m, 3H), 7.11 (dd, J = 8.4 and 2.8 Hz, 7H) [7.12 (dd, J = 8.8 and 2.8 Hz, 1H)], 7.00 (dd, J = 4.8 and 3.6 Hz, 1H), 6.95 (td, J = 8.4 and 1.0 Hz, 2H) [7.06 (td, J = 8.4 and 1.0 Hz, 2H)], 6.89 (dd, J = 3.6 and 1.2 Hz, 1H), 6.82 (s, 1H) [6.52 (s, 1H)], 3.93 (s, 3H) [3.94 (s, 3H)]; LC-MS (ESI, m/z) 407.37 (M + H+, 100). Anal. calcd for C23H15FO4S: C, 67.97; H, 3.72; S, 7.89. Found: C, 67.93; H, 3.78; S, 7.87.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1651 (C
O), 1651 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1592, 1193 (C–O–C), 964, 719; 1H-NMR (400 MHz, CDCl3) δ 8.13 (dd, J = 6.4 and 2.4 Hz, 1H), 8.01 (dd, J = 6.4 and 2.4 Hz, 1H), 7.68–7.72 (m, 2H), 7.30–7.39 (m, 6H), 7.16 (dd, J = 3.6 and 0.8 Hz, 1H), 7.02 (dd, J = 4.8 and 3.6 Hz, 1H), 6.01 (d, J = 6.0 Hz, 1H), 4.87 (d, J = 6.0 Hz, 1H); 13C-NMR (100 MHz, CDCl3) δ 181.7, 178.3, 159.1, 141.3, 139.8, 134.6, 133.3, 131.9, 129.4, 128.2, 127.6, 127.4, 127.0, 126.6, 126.5, 126.0, 90.8, 55.4; LC/MS, (ESI, m/z): 359.02 (M + H+, 100). Anal. calcd for C22H14O3S: C, 73.72; H, 3.94; S, 8.95. Found: C, 73.83; H, 3.89; S, 8.88.
O), 1592, 1193 (C–O–C), 964, 719; 1H-NMR (400 MHz, CDCl3) δ 8.13 (dd, J = 6.4 and 2.4 Hz, 1H), 8.01 (dd, J = 6.4 and 2.4 Hz, 1H), 7.68–7.72 (m, 2H), 7.30–7.39 (m, 6H), 7.16 (dd, J = 3.6 and 0.8 Hz, 1H), 7.02 (dd, J = 4.8 and 3.6 Hz, 1H), 6.01 (d, J = 6.0 Hz, 1H), 4.87 (d, J = 6.0 Hz, 1H); 13C-NMR (100 MHz, CDCl3) δ 181.7, 178.3, 159.1, 141.3, 139.8, 134.6, 133.3, 131.9, 129.4, 128.2, 127.6, 127.4, 127.0, 126.6, 126.5, 126.0, 90.8, 55.4; LC/MS, (ESI, m/z): 359.02 (M + H+, 100). Anal. calcd for C22H14O3S: C, 73.72; H, 3.94; S, 8.95. Found: C, 73.83; H, 3.89; S, 8.88.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1651 (C
O), 1651 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1361, 1209 (C–O–C), 962, 728; 1H-NMR (400 MHz, CDCl3) δ 8.15 (m, 1H), 8.00 (m, 1H), 7.70 (m, 2H), 7.32–7.39 (m, 3H), 7.29 (dd, J = 5.2 and 1.2 Hz, 1H), 7.20 (d, J = 6.4 Hz, 2H), 7.15 (dd, J = 3.6 and 1.2 Hz, 1H), 6.99 (dd, J = 5.2 and 3.6 Hz, 1H), 4.98 (s, 1H), 1.48 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 181.8, 178.4, 159.4, 149.5, 136.2, 134.6, 133.4, 131.9, 129.1, 128.9, 128.4, 127.3, 126.7, 126.6, 125.9, 125.5, 123.8, 94.9, 58.5, 26.1; GC-MS (m/z, %) 372.2 (M+, 100). Anal. calcd for C23H16O3S: C, 74.17; H, 4.33; S, 8.61. Found: C, 74.18; H, 4.22; S, 8.63.
O), 1361, 1209 (C–O–C), 962, 728; 1H-NMR (400 MHz, CDCl3) δ 8.15 (m, 1H), 8.00 (m, 1H), 7.70 (m, 2H), 7.32–7.39 (m, 3H), 7.29 (dd, J = 5.2 and 1.2 Hz, 1H), 7.20 (d, J = 6.4 Hz, 2H), 7.15 (dd, J = 3.6 and 1.2 Hz, 1H), 6.99 (dd, J = 5.2 and 3.6 Hz, 1H), 4.98 (s, 1H), 1.48 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 181.8, 178.4, 159.4, 149.5, 136.2, 134.6, 133.4, 131.9, 129.1, 128.9, 128.4, 127.3, 126.7, 126.6, 125.9, 125.5, 123.8, 94.9, 58.5, 26.1; GC-MS (m/z, %) 372.2 (M+, 100). Anal. calcd for C23H16O3S: C, 74.17; H, 4.33; S, 8.61. Found: C, 74.18; H, 4.22; S, 8.63.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1660 (C
O), 1660 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1635, 1225 (C–O–C), 964, 699; 1H-NMR (400 MHz, CDCl3) δ 8.19 (m, 1H), 8.01 (m, 1H), 7.73 (m, 2H), 7.08 (m, 3H), 7.01 (d, J = 5.2 Hz, 1H), 6.91 (m, 2H), 6.69 (dd, J = 5.2 and 4.0 Hz, 1H), 6.59 (d, J = 4.0 Hz, 1H), 4.66 (s, 1H), 2.07 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 181.9, 178.4, 159.1, 143.2, 136.7, 134.6, 133.4, 133.3, 131.9, 128.7, 128.3, 127.7, 126.7, 126.6, 126.3, 125.2, 125.1, 96.4, 59.1, 30.8; GC-MS (m/z, %) 372.2 (M+, 100). Anal. calcd for C23H16O3S: C, 74.17; H, 4.33; S, 8.61. Found: C, 74.10; H, 4.32; S, 8.67.
O), 1635, 1225 (C–O–C), 964, 699; 1H-NMR (400 MHz, CDCl3) δ 8.19 (m, 1H), 8.01 (m, 1H), 7.73 (m, 2H), 7.08 (m, 3H), 7.01 (d, J = 5.2 Hz, 1H), 6.91 (m, 2H), 6.69 (dd, J = 5.2 and 4.0 Hz, 1H), 6.59 (d, J = 4.0 Hz, 1H), 4.66 (s, 1H), 2.07 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 181.9, 178.4, 159.1, 143.2, 136.7, 134.6, 133.4, 133.3, 131.9, 128.7, 128.3, 127.7, 126.7, 126.6, 126.3, 125.2, 125.1, 96.4, 59.1, 30.8; GC-MS (m/z, %) 372.2 (M+, 100). Anal. calcd for C23H16O3S: C, 74.17; H, 4.33; S, 8.61. Found: C, 74.10; H, 4.32; S, 8.67.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1645 (C
O), 1645 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1628, 1208 (C–O–C), 968, 710; 1H-NMR (400 MHz, CDCl3) δ 8.09 (t, J = 8.0 Hz, 2H), 7.73 (td, J = 7.6 and 1.6 Hz, 1H), 7.70 (td, J = 7.6 and 1.6 Hz, 1H), 7.30 (d, J = 4.8 Hz, 1H), 7.10 (d, J = 3.2 Hz, 1H), 6.99 (dd, J = 4.8 and 3.2 Hz, 1H), 4.06 (dd, J = 8.8 and 1.6 Hz, 1H), 2.73 (dd, J = 14.4 and 6.0 Hz, 1H), 2.20 (m, 3H), 1.96 (m, 1H), 1.81 (m, 1H); 13C-NMR (100 MHz, CDCl3) δ 182.5, 178.0, 159.0, 145.7, 134.4, 133.4, 133.2, 131.9, 127.3, 126.6, 126.3, 125.9, 124.3, 102.2, 53.3, 42.6, 33.1, 25.2; GC-MS (m/z, %) 322.0 (M+, 100). Anal. calcd for C19H14O3S: C, 70.79; H, 4.38; S, 9.95. Found: C, 70.65; H, 4.41; S, 9.90.
O), 1628, 1208 (C–O–C), 968, 710; 1H-NMR (400 MHz, CDCl3) δ 8.09 (t, J = 8.0 Hz, 2H), 7.73 (td, J = 7.6 and 1.6 Hz, 1H), 7.70 (td, J = 7.6 and 1.6 Hz, 1H), 7.30 (d, J = 4.8 Hz, 1H), 7.10 (d, J = 3.2 Hz, 1H), 6.99 (dd, J = 4.8 and 3.2 Hz, 1H), 4.06 (dd, J = 8.8 and 1.6 Hz, 1H), 2.73 (dd, J = 14.4 and 6.0 Hz, 1H), 2.20 (m, 3H), 1.96 (m, 1H), 1.81 (m, 1H); 13C-NMR (100 MHz, CDCl3) δ 182.5, 178.0, 159.0, 145.7, 134.4, 133.4, 133.2, 131.9, 127.3, 126.6, 126.3, 125.9, 124.3, 102.2, 53.3, 42.6, 33.1, 25.2; GC-MS (m/z, %) 322.0 (M+, 100). Anal. calcd for C19H14O3S: C, 70.79; H, 4.38; S, 9.95. Found: C, 70.65; H, 4.41; S, 9.90.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1639 (C
O), 1639 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1592, 1275 (C–O–C), 725; 1H-NMR (400 MHz, CDCl3) δ 8.15 (m, 2H), 7.80 (td, J = 7.6 and 1.2 Hz, 1H), 7.74 (td, J = 7.6 and 1.2 Hz, 1H), 7.45 (s, 1H, OH), 7.09 (d, J = 4.8 Hz, 1H), 6.97 (d, J = 3.6 Hz, 1H), 6.91 (dd, J = 4.8 and 3.6 Hz, 1H), 3.04 (s, 2H), 2.82 (m, 2H), 2.16 (m, 2H); 13C-NMR (100 MHz, CDCl3) δ 183.4, 181.3, 153.4, 139.5, 136.3, 135.4, 133.6, 133.3, 129.7, 127.5, 127.4, 126.9, 126.5, 125.9, 125.6, 122.0, 38.5, 37.3, 22.9; GC/MS, (m/z, %): 322.0 (M+, 45). Anal. calcd for C19H14O3S: C, 70.79; H, 4.38; S, 9.95. Found: C, 70.90; H, 4.26; S, 10.03.
O), 1592, 1275 (C–O–C), 725; 1H-NMR (400 MHz, CDCl3) δ 8.15 (m, 2H), 7.80 (td, J = 7.6 and 1.2 Hz, 1H), 7.74 (td, J = 7.6 and 1.2 Hz, 1H), 7.45 (s, 1H, OH), 7.09 (d, J = 4.8 Hz, 1H), 6.97 (d, J = 3.6 Hz, 1H), 6.91 (dd, J = 4.8 and 3.6 Hz, 1H), 3.04 (s, 2H), 2.82 (m, 2H), 2.16 (m, 2H); 13C-NMR (100 MHz, CDCl3) δ 183.4, 181.3, 153.4, 139.5, 136.3, 135.4, 133.6, 133.3, 129.7, 127.5, 127.4, 126.9, 126.5, 125.9, 125.6, 122.0, 38.5, 37.3, 22.9; GC/MS, (m/z, %): 322.0 (M+, 45). Anal. calcd for C19H14O3S: C, 70.79; H, 4.38; S, 9.95. Found: C, 70.90; H, 4.26; S, 10.03.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1651 (C
O), 1651 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1633, 1192 (C–O–C), 964, 719; 1H-NMR (400 MHz, CDCl3) δ 8.02 (t, J = 8.0 Hz, 2H), 7.72 (td, J = 7.6 and 1.6 Hz, 1H), 7.67 (td, J = 7.6 and 1.6 Hz, 1H), 7.28 (dd, J = 5.2 and 0.8 Hz, 1H), 7.15 (dd, J = 3.6 and 0.8 Hz, 1H), 6.98 (dd, J = 5.2 and 3.6 Hz, 1H), 3.86 (t, J = 6.4 Hz, 1H), 2.39 (m, 1H), 2.22 (m, 1H), 2.05 (m, 2H), 1.62 (m, 4H); 13C-NMR (100 MHz, CDCl3) δ 182.7, 178.4, 159.0, 147.8, 134.4, 133.4, 133.2, 131.8, 127.9, 127.1, 126.1, 126.2, 125.6, 124.5, 93.2, 47.1, 34.3, 25.2, 19.6, 19.4; GC/MS, (m/z, %): 336 (M+, 100). Anal. calcd for C20H16O3S: C, 71.41; H, 4.79; S, 9.53. Found: C, 71.39; H, 4.72; S, 9.65.
O), 1633, 1192 (C–O–C), 964, 719; 1H-NMR (400 MHz, CDCl3) δ 8.02 (t, J = 8.0 Hz, 2H), 7.72 (td, J = 7.6 and 1.6 Hz, 1H), 7.67 (td, J = 7.6 and 1.6 Hz, 1H), 7.28 (dd, J = 5.2 and 0.8 Hz, 1H), 7.15 (dd, J = 3.6 and 0.8 Hz, 1H), 6.98 (dd, J = 5.2 and 3.6 Hz, 1H), 3.86 (t, J = 6.4 Hz, 1H), 2.39 (m, 1H), 2.22 (m, 1H), 2.05 (m, 2H), 1.62 (m, 4H); 13C-NMR (100 MHz, CDCl3) δ 182.7, 178.4, 159.0, 147.8, 134.4, 133.4, 133.2, 131.8, 127.9, 127.1, 126.1, 126.2, 125.6, 124.5, 93.2, 47.1, 34.3, 25.2, 19.6, 19.4; GC/MS, (m/z, %): 336 (M+, 100). Anal. calcd for C20H16O3S: C, 71.41; H, 4.79; S, 9.53. Found: C, 71.39; H, 4.72; S, 9.65.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1646 (C
O), 1646 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1635, 1265 (C–O–C), 720; 1H-NMR (400 MHz, CDCl3) δ 8.10 (d, J = 7.2 Hz, 2H), 7.77 (t, J = 7.6 Hz, 1H), 7.71 (t, J = 7.6 Hz, 1H), 7.01 (d, J = 4.4 Hz, 1H), 6.92 (d, J = 3.6 Hz, 1H), 6.82 (dd, J = 4.8 and 3.6 Hz, 1H), 2.60–2.64 (m, 2H), 2.29–2.31 (m, 2H), 1.79–1.92 (m, 4H); LC/MS (ESI, m/z): 337.38 (MH+, 85). Anal. calcd for C20H16O3S: C, 71.41; H, 4.79; S, 9.53. Found: C, 71.32; H, 4.85; S, 9.44.
O), 1635, 1265 (C–O–C), 720; 1H-NMR (400 MHz, CDCl3) δ 8.10 (d, J = 7.2 Hz, 2H), 7.77 (t, J = 7.6 Hz, 1H), 7.71 (t, J = 7.6 Hz, 1H), 7.01 (d, J = 4.4 Hz, 1H), 6.92 (d, J = 3.6 Hz, 1H), 6.82 (dd, J = 4.8 and 3.6 Hz, 1H), 2.60–2.64 (m, 2H), 2.29–2.31 (m, 2H), 1.79–1.92 (m, 4H); LC/MS (ESI, m/z): 337.38 (MH+, 85). Anal. calcd for C20H16O3S: C, 71.41; H, 4.79; S, 9.53. Found: C, 71.32; H, 4.85; S, 9.44.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1633 (C
O), 1633 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1593, 1253 (C–O–C), 955, 757; 1H-NMR (400 MHz, CDCl3) δ 8.12 (dd, J = 7.2 and 1.6 Hz, 1H), 8.07 (dd, J = 7.2 and 1.6 Hz, 1H), 7.72 (td, J = 7.2 and 1.6 Hz, 1H), 7.69 (td, J = 7.2 and 1.6 Hz, 1H), 7.51–7.54 (m, 2H), 7.35–7.43 (m, 4H), 6.31 (dd, J = 3.2 and 2.0 Hz, 1H), 6.11 (d, J = 2.8 Hz, 1H), 4.13 (d, J = 17.2 Hz, 1H), 3.64 (d, J = 17.2 Hz, 1H); 13C-NMR (100 MHz, CDCl3) δ 182.4, 177.9, 158.6, 154.1, 144.1, 141.5, 134.4, 133.3, 133.2, 131.9, 128.8, 126.6, 126.3, 125.8, 123.7, 110.8, 110.7, 91.4, 40.2; LC/MS, (ESI, m/z): 343.41 (M + H+, 100). Anal. calcd for C22H14O4: C, 77.18; H, 4.12. Found: C, 77.23; H, 4.10.
O), 1593, 1253 (C–O–C), 955, 757; 1H-NMR (400 MHz, CDCl3) δ 8.12 (dd, J = 7.2 and 1.6 Hz, 1H), 8.07 (dd, J = 7.2 and 1.6 Hz, 1H), 7.72 (td, J = 7.2 and 1.6 Hz, 1H), 7.69 (td, J = 7.2 and 1.6 Hz, 1H), 7.51–7.54 (m, 2H), 7.35–7.43 (m, 4H), 6.31 (dd, J = 3.2 and 2.0 Hz, 1H), 6.11 (d, J = 2.8 Hz, 1H), 4.13 (d, J = 17.2 Hz, 1H), 3.64 (d, J = 17.2 Hz, 1H); 13C-NMR (100 MHz, CDCl3) δ 182.4, 177.9, 158.6, 154.1, 144.1, 141.5, 134.4, 133.3, 133.2, 131.9, 128.8, 126.6, 126.3, 125.8, 123.7, 110.8, 110.7, 91.4, 40.2; LC/MS, (ESI, m/z): 343.41 (M + H+, 100). Anal. calcd for C22H14O4: C, 77.18; H, 4.12. Found: C, 77.23; H, 4.10.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1590, 1331 (C–O–C), 724; 1H-NMR (400 MHz, CDCl3) δ 8.00 (dd, J = 7.6 and 1.6 Hz, 2H) [8.08 (td, J = 7.6 and 1.6 Hz, 2H)], 7.66–7.76 (m, 1H + [2H]), 7.63 (td, J = 7.6 and 1.6 Hz, 1H), 7.49–7.51 (m, 2H + [2H]), 7.23–7.34 (m, 4H + [4H]), 7.03 (s, 1H) [6.48 (s, 1H)], 6.38 (m, 1H + [1H]), 6.15 (d, J = 3.6 Hz, 1H) [6.30 (d, J = 3.2 Hz, 1H)]; LC/MS, (ESI, m/z): 343.55 (M + H+, 12), 365.44 (M + Na+, 24), 406.45 (M + Na+ + CH3CN, 100). Anal. calcd for C22H14O4: C, 77.18; H, 4.12. Found: C, 77.34, H, 4.08.
O), 1590, 1331 (C–O–C), 724; 1H-NMR (400 MHz, CDCl3) δ 8.00 (dd, J = 7.6 and 1.6 Hz, 2H) [8.08 (td, J = 7.6 and 1.6 Hz, 2H)], 7.66–7.76 (m, 1H + [2H]), 7.63 (td, J = 7.6 and 1.6 Hz, 1H), 7.49–7.51 (m, 2H + [2H]), 7.23–7.34 (m, 4H + [4H]), 7.03 (s, 1H) [6.48 (s, 1H)], 6.38 (m, 1H + [1H]), 6.15 (d, J = 3.6 Hz, 1H) [6.30 (d, J = 3.2 Hz, 1H)]; LC/MS, (ESI, m/z): 343.55 (M + H+, 12), 365.44 (M + Na+, 24), 406.45 (M + Na+ + CH3CN, 100). Anal. calcd for C22H14O4: C, 77.18; H, 4.12. Found: C, 77.34, H, 4.08.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1656 (C
O), 1656 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1591, 1240 (C–O–C), 954, 741; 1H-NMR (400 MHz, CDCl3) δ 7.95 (d, J = 7.6 Hz, 1H), 7.90 (s, 1H), 7.49–7.53 (m, 3H), 7.36–7.43 (m, 4H), 6.31 (m, 1H), 6.11 (d, J = 2.8 Hz, 1H), 4.11 (d, J = 17.2 Hz, 1H), 3.62 (d, J = 17.2 Hz, 1H), 2.47 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 182.2, 177.9, 158.2, 153.9, 144.1, 143.8, 141.3, 134.7, 131.5, 130.7, 128.5, 126.9, 126.2, 125.5, 123.4, 110.5, 110.4, 91.1, 40.0, 21.6; LC/MS, (ESI, m/z) 357.5 (M + H+, 20), 379.5 (M + Na+, 30), 420.5 (M + Na+ + CH3CN, 100). Anal. calcd for C23H16O4: C, 77.52; H, 4.53. Found: C, 77.57; H, 4.53.
O), 1591, 1240 (C–O–C), 954, 741; 1H-NMR (400 MHz, CDCl3) δ 7.95 (d, J = 7.6 Hz, 1H), 7.90 (s, 1H), 7.49–7.53 (m, 3H), 7.36–7.43 (m, 4H), 6.31 (m, 1H), 6.11 (d, J = 2.8 Hz, 1H), 4.11 (d, J = 17.2 Hz, 1H), 3.62 (d, J = 17.2 Hz, 1H), 2.47 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 182.2, 177.9, 158.2, 153.9, 144.1, 143.8, 141.3, 134.7, 131.5, 130.7, 128.5, 126.9, 126.2, 125.5, 123.4, 110.5, 110.4, 91.1, 40.0, 21.6; LC/MS, (ESI, m/z) 357.5 (M + H+, 20), 379.5 (M + Na+, 30), 420.5 (M + Na+ + CH3CN, 100). Anal. calcd for C23H16O4: C, 77.52; H, 4.53. Found: C, 77.57; H, 4.53.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1588, 1254 (C–O–C), 754; 1H-NMR (400 MHz, CDCl3) δ 7.90 (d, J = 7.6 Hz, 1H) [7.97 (d, J = 8.0 Hz, 1H)], 7.80 (s, 1H) [7.89 (s, 1H)], 7.49–7.54 (m, 3H + [3H]), 7.24–7.38 (m, 4H + [4H]), 7.18 (s, 1H + [1H], OH), 7.02 (s, 1H) [6.48 (s, 1H)], 6.37–6.40 (m, 1H + [1H]), 6.15 (d, J = 2.8 Hz, 1H) [6.30 (d, J = 3.6 Hz, 1H)], 2.45 (s, 3H) [2.49 (s, 3H)]; LC/MS, (ESI, m/z): 357.5 (M + H, 10), 379.5 (M + Na, 37), 420.5 (M + Na + CH3CN, 100). Anal. calcd for C23H16O4: C, 77.52; H, 4.53. Found: C, 77.48; H, 4.59.
O), 1588, 1254 (C–O–C), 754; 1H-NMR (400 MHz, CDCl3) δ 7.90 (d, J = 7.6 Hz, 1H) [7.97 (d, J = 8.0 Hz, 1H)], 7.80 (s, 1H) [7.89 (s, 1H)], 7.49–7.54 (m, 3H + [3H]), 7.24–7.38 (m, 4H + [4H]), 7.18 (s, 1H + [1H], OH), 7.02 (s, 1H) [6.48 (s, 1H)], 6.37–6.40 (m, 1H + [1H]), 6.15 (d, J = 2.8 Hz, 1H) [6.30 (d, J = 3.6 Hz, 1H)], 2.45 (s, 3H) [2.49 (s, 3H)]; LC/MS, (ESI, m/z): 357.5 (M + H, 10), 379.5 (M + Na, 37), 420.5 (M + Na + CH3CN, 100). Anal. calcd for C23H16O4: C, 77.52; H, 4.53. Found: C, 77.48; H, 4.59.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1651 (C
O), 1651 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1599, 1242 (C–O–C), 758; 1H-NMR (400 MHz, CDCl3) δ 7.84 (d, J = 1.6 Hz, 1H), 7.51–7.53 (m, 2H), 7.42 (dd, J = 2.0 and 0.8 Hz, 1H), 7.34–7.41 (m, 3H), 7.27 (s, 1H), 6.30 (dd, J = 3.2 and 2.0 Hz, 1H), 6.12 (dd, J = 2.8 and 0.8 Hz, 1H), 4.08 (d, J = 17.2 Hz, 1H), 3.58 (d, J = 17.2 Hz, 1H), 2.69 (s, 3H), 2.40 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 184.9, 178.3, 157.0, 154.3, 144.0, 143.1, 141.7, 141.4, 139.3, 133.5, 128.7, 128.6, 128.0, 126.3, 125.8, 125.0, 110.7, 110.6, 91.0, 40.6, 23.0, 21.6; LC/MS, (ESI, m/z): 371.5 (M + H+, 48), 393.7 (M + Na+, 36), 434.6 (M + Na+ + CH3CN, 100). Anal. calcd for C24H18O4: C, 77.82; H, 4.90. Found: C, 77.71; H, 4.91.
O), 1599, 1242 (C–O–C), 758; 1H-NMR (400 MHz, CDCl3) δ 7.84 (d, J = 1.6 Hz, 1H), 7.51–7.53 (m, 2H), 7.42 (dd, J = 2.0 and 0.8 Hz, 1H), 7.34–7.41 (m, 3H), 7.27 (s, 1H), 6.30 (dd, J = 3.2 and 2.0 Hz, 1H), 6.12 (dd, J = 2.8 and 0.8 Hz, 1H), 4.08 (d, J = 17.2 Hz, 1H), 3.58 (d, J = 17.2 Hz, 1H), 2.69 (s, 3H), 2.40 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 184.9, 178.3, 157.0, 154.3, 144.0, 143.1, 141.7, 141.4, 139.3, 133.5, 128.7, 128.6, 128.0, 126.3, 125.8, 125.0, 110.7, 110.6, 91.0, 40.6, 23.0, 21.6; LC/MS, (ESI, m/z): 371.5 (M + H+, 48), 393.7 (M + Na+, 36), 434.6 (M + Na+ + CH3CN, 100). Anal. calcd for C24H18O4: C, 77.82; H, 4.90. Found: C, 77.71; H, 4.91.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1640, 1334, 1091 (C–O–C), 756; 1H-NMR (400 MHz, CDCl3) δ 7.74 (s, 1H) [7.83 (s, 1H)], 7.47–7.51 (m, 2H + [2H]), 7.24–7.37 (m, 5H + [4H]), [7.15 (s, 1H)], 7.06 (s, 1H) [6.50 (s, 1H)], 6.39 (m, 1H + [1H]), 6.17 (d, J = 3.2 Hz, 1H) [6.30 (d, J = 3.6 Hz, 1H)], 2.38 (s, 3H), 2.37 (s, 3H) [2.43 (s, 3H), 2.54 (s, 3H)]; LC/MS, (ESI, m/z): 371.5 (MH+, 35), 393.6 (M + Na, 40), 434.5 (M + Na + CH3CN, 100). Anal. calcd for C24H18O4: C, 77.82; H, 4.90. Found: C, 77.69; H, 4.99.
O), 1640, 1334, 1091 (C–O–C), 756; 1H-NMR (400 MHz, CDCl3) δ 7.74 (s, 1H) [7.83 (s, 1H)], 7.47–7.51 (m, 2H + [2H]), 7.24–7.37 (m, 5H + [4H]), [7.15 (s, 1H)], 7.06 (s, 1H) [6.50 (s, 1H)], 6.39 (m, 1H + [1H]), 6.17 (d, J = 3.2 Hz, 1H) [6.30 (d, J = 3.6 Hz, 1H)], 2.38 (s, 3H), 2.37 (s, 3H) [2.43 (s, 3H), 2.54 (s, 3H)]; LC/MS, (ESI, m/z): 371.5 (MH+, 35), 393.6 (M + Na, 40), 434.5 (M + Na + CH3CN, 100). Anal. calcd for C24H18O4: C, 77.82; H, 4.90. Found: C, 77.69; H, 4.99.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1659 (C
O), 1659 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1597, 1229 (C–O–C), 984, 753; 1H-NMR (400 MHz, CDCl3) δ 8.03 (d, J = 8.8 Hz, 1H), 7.51–7.53 (m, 3H), 7.36–7.43 (m, 4H), 7.10 (dd, J = 8.8 and 2.8 Hz, 1H), 6.31 (dd, J = 3.2 and 2.0 Hz, 1H), 6.11 (d, J = 3.2 Hz, 1H), 4.10 (d, J = 17.2 Hz, 1H), 3.93 (s, 3H), 3.60 (d, J = 17.2 Hz, 1H); 13C-NMR (100 MHz, CDCl3) δ 182.2, 176.9, 164.8, 159.1, 154.1, 144.1, 141.6, 135.6, 129.1, 128.7, 125.8, 125.2, 123.0, 118.7, 110.8, 110.7, 91.5, 56.2, 40.2; LC/MS, (ESI, m/z) 373.4 (M + H+, 100). Anal. calcd for C23H16O4: C, 74.19; H, 4.33. Found: C, 74.25; H, 4.34.
O), 1597, 1229 (C–O–C), 984, 753; 1H-NMR (400 MHz, CDCl3) δ 8.03 (d, J = 8.8 Hz, 1H), 7.51–7.53 (m, 3H), 7.36–7.43 (m, 4H), 7.10 (dd, J = 8.8 and 2.8 Hz, 1H), 6.31 (dd, J = 3.2 and 2.0 Hz, 1H), 6.11 (d, J = 3.2 Hz, 1H), 4.10 (d, J = 17.2 Hz, 1H), 3.93 (s, 3H), 3.60 (d, J = 17.2 Hz, 1H); 13C-NMR (100 MHz, CDCl3) δ 182.2, 176.9, 164.8, 159.1, 154.1, 144.1, 141.6, 135.6, 129.1, 128.7, 125.8, 125.2, 123.0, 118.7, 110.8, 110.7, 91.5, 56.2, 40.2; LC/MS, (ESI, m/z) 373.4 (M + H+, 100). Anal. calcd for C23H16O4: C, 74.19; H, 4.33. Found: C, 74.25; H, 4.34.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1598, 1336, 917, 747; 1H-NMR (400 MHz, CDCl3) δ 7.95 (d, J = 8.8 Hz, 1H) [8.04 (d, J = 8.8 Hz, 1H)], 7.49–7.51 (m, 2H + [2H]), 7.46 (d, J = 2.8 Hz, 1H) [7.54 (d, J = 2.8 Hz, 1H)], 7.25–7.39 (m, 4H + [4H]), 7.08 (dd, J = 8.8 and 2.8 Hz, 1H) [7.14 (dd, J = 8.8 and 2.8 Hz, 1H)], 6.99 (s, 1H) [6.46 (s, 1H)], 6.40 (m, 1H + [1H]), 6.14 (d, J = 3.2 Hz, 1H) [6.29 (d, J = 3.2 Hz, 1H)], 3.92 (s, 3H) [3.94 (s, 3H)]; LC/MS, (ESI, m/z): 373.4 (M + H+, 20), 395.5 (M + Na+, 37), 436.5 (M + Na+ + CH3CN, 100). Anal. calcd for C23H16O4: C, 74.19; H, 4.33. Found: C, 74.29; H, 4.22.
O), 1598, 1336, 917, 747; 1H-NMR (400 MHz, CDCl3) δ 7.95 (d, J = 8.8 Hz, 1H) [8.04 (d, J = 8.8 Hz, 1H)], 7.49–7.51 (m, 2H + [2H]), 7.46 (d, J = 2.8 Hz, 1H) [7.54 (d, J = 2.8 Hz, 1H)], 7.25–7.39 (m, 4H + [4H]), 7.08 (dd, J = 8.8 and 2.8 Hz, 1H) [7.14 (dd, J = 8.8 and 2.8 Hz, 1H)], 6.99 (s, 1H) [6.46 (s, 1H)], 6.40 (m, 1H + [1H]), 6.14 (d, J = 3.2 Hz, 1H) [6.29 (d, J = 3.2 Hz, 1H)], 3.92 (s, 3H) [3.94 (s, 3H)]; LC/MS, (ESI, m/z): 373.4 (M + H+, 20), 395.5 (M + Na+, 37), 436.5 (M + Na+ + CH3CN, 100). Anal. calcd for C23H16O4: C, 74.19; H, 4.33. Found: C, 74.29; H, 4.22.Acknowledgements
The authors are indebted to the Scientific and Technological Research Council of Turkey (TUBITAK, Grant 110T493), and O. Alagöz thanks the TÜBİTAK for 2214-doctoral fellowship.Notes and references
- (a) M. Mondal and U. Bora, RSC Adv., 2013, 3(41), 18716–18754 RSC; (b) B. B. Snider, Chem. Rev., 1996, 96, 339–363 CrossRef CAS PubMed; (c) J. Iqbal, B. Bhatia and N. K. Nayyar, Chem. Rev., 1994, 94, 519–564 CrossRef CAS; (d) J. W. Burton, Encyclopedia of Radicals in Chemistry, Biology and Materials, 2012, vol. 2, pp. 901–941 Search PubMed; (e) A. S. Demir and M. Emrullahoglu, Curr. Org. Synth., 2007, 4, 321–350 CrossRef CAS.
- (a) O. Alagoz, M. Yılmaz and A. T. Pekel, Synth. Commun., 2006, 36, 1005–1013 CrossRef CAS; (b) M. Yılmaz and A. T. Pekel, Synth. Commun., 2001, 31, 3871–3876 CrossRef PubMed.
- (a) M. Yılmaz and A. T. Pekel, Synth. Commun., 2001, 31, 2189–2194 CrossRef PubMed; (b) R. Caliskan, T. Pekel, W. H. Watson and M. Balci, Tetrahedron Lett., 2005, 46, 6227–6230 CrossRef CAS PubMed; (c) C. Dengiz, R. Çalışkan and M. Balci, Tetrahedron Lett., 2012, 53, 550–552 CrossRef CAS PubMed; (d) M. Yılmaz, Tetrahedron, 2011, 67, 8255–8263 CrossRef PubMed; (e) E. V. Burgaz, M. Yilmaz, A. T. Pekel and A. Oktemer, Tetrahedron, 2007, 63, 7229–7239 CrossRef CAS PubMed; (f) M. Yılmaz, N. Uzunalioglu, M. Yakut and A. T. Pekel, Turk. J. Chem., 2008, 32, 411–422 Search PubMed; (g) M. Yilmaz and A. T. Pekel, J. Fluorine Chem., 2011, 132, 628–635 CrossRef CAS PubMed; (h) M. Yilmaz, Helv. Chim. Acta, 2011, 94, 1335–1342 CrossRef CAS PubMed; (i) C. Curti, M. D. Crozet and P. Vanelle, Tetrahedron, 2009, 65, 200–205 CrossRef CAS PubMed; (j) B. Gregory, A. F. Parsons and C. B. Thomas, Tetrahedron Lett., 2000, 41, 7751–7755 CrossRef; (k) Y. Ito, S. Jogo, N. Fukuda, R. Okumura and H. Nishino, Synthesis, 2011, 9, 1365–1374 Search PubMed; (l) Y. R. Lee and B. S. Kim, Synth. Commun., 2003, 33, 4123–4135 CrossRef CAS PubMed; (m) E. Bicer, M. Yilmaz, E. V. Burgaz and A. T. Pekel, Helv. Chim. Acta, 2013, 96(1), 135–141 CrossRef CAS PubMed; (n) E. Bicer, M. Yilmaz, M. Karataş and A. T. Pekel, Helv. Chim. Acta, 2012, 95(5), 795–804 CrossRef CAS PubMed.
- (a) A. Bouhlel, C. Curti, M. P. Bertrand and P. Vanelle, Tetrahedron, 2012, 68, 3596–3604 CrossRef CAS PubMed; (b) C.-P. Chuang and A.-I. Tsai, Tetrahedron, 2007, 63, 9712–9717 CrossRef CAS PubMed; (c) M. A. Haque and H. Nishino, Heterocycles, 2011, 83, 1783–1805 CrossRef CAS; (d) L. Curry, M. S. Hallside, L. H. Powell, S. J. Sprague and J. W. Burton, Tetrahedron, 2009, 65, 10882–10892 CrossRef CAS PubMed; (e) F.-B. Li, T.-X. Liu, Y.-S. Huang and G.-W. Wang, J. Org. Chem., 2009, 74, 7743–7749 CrossRef CAS PubMed.
- (a) J. Cossy and C. Leblanc, Tetrahedron Lett., 1989, 30, 4531–4534 CrossRef CAS; (b) J. B. Bremner and W. Jaturonrusmee, Aust. J. Chem., 1990, 43, 1461–1467 CrossRef CAS.
- (a) N. S. Simpkins and M. D. Weller, Tetrahedron Lett., 2010, 51, 4823–4826 CrossRef CAS PubMed; (b) J. J. Davies, T. M. Krulle and J. W. Burton, Org. Lett., 2010, 12, 2738–2741 CrossRef CAS PubMed; (c) A. F. Parsons and D. A. J. Williams, Tetrahedron, 2000, 56, 7217–7228 CrossRef CAS.
- (a) R. H. Thomson, Natural Occuring Quinones IV: Recent Advances, Chapman and Hall, London, 1997 Search PubMed; (b) Z.-Y. Lin, Y.-L. Chen, C.-S. Lee and C.-P. Chuang, Eur. J. Org. Chem., 2010, 20, 3876–3882 CrossRef PubMed.
- (a) E. N. Silva Jr, M. C. B. V. Souza, A. V. Pinto, M. C. Pinto, M. O. O. Goulart, F. W. A. Barros, C. Pessoa, L. V. Costa-Lotufo, R. C. Montenegro, M. O. Moraes and V. F. Ferreira, Bioorg. Med. Chem., 2007, 15, 7035–7041 CrossRef PubMed; (b) E. N. Silva Jr, C. F. Deus, B. C. Cavalcanti, C. Pessoa, L. V. Costa-Lotufo, R. C. Montenegro, M. O. Moraes, M. C. Pinto, C. A. Simone, V. F. Ferreira, M. O. F. Goulart, C. K. Z. Andrade and A. V. Pinto, J. Med. Chem., 2010, 53, 504–508 CrossRef PubMed.
- E. N. Silva Jr, R. F. Menna-Barreto, M. C. Pinto, R. S. Silva, D. V. Teixeira, M. C. B. V. Souza, C. A. De Simone, S. L. De Castro, V. F. Ferreira and A. V. Pinto, Eur. J. Med. Chem., 2008, 43, 1774–1780 CrossRef PubMed.
- A. F. Santos, P. A. L. Ferraz, F. C. de Abreu, E. Chiari, M. O. F. Goulart and A. E. G. Sant'Ana, Planta Med., 2001, 67, 92–95 CrossRef CAS PubMed.
- M. J. Teixeira, Y. M. de Almeida, J. R. Viana, J. G. Holanda, T. P. Rodrigues, J. R. C. Prata, I. V. B. Coelho, V. S. Rao and M. M. L. Pompeu, Phytother. Res., 2001, 15, 44–48 CrossRef CAS.
- A. P. Neves, C. C. Barbosa, S. J. Greco, M. D. Vargas, L. C. Visentin, C. B. Pinheiro, A. S. Mangrich, J. P. Barbosa and G. L. Costa, J. Braz. Chem. Soc., 2009, 20, 712–727 CrossRef CAS PubMed.
- S. B. Ferreira, F. C. da Silva, F. A. F. M. Bezerra, M. C. S. Lourenco, C. R. Kaiser, A. C. Pinto and V. F. Ferreira, Arch. Pharm., 2010, 343, 81–90 CAS.
- C. P. V. Freire, S. B. Ferreira, N. S. Melo de Oliveira, A. B. J. Matsuura, I. L. Gama, F. C. da Silva, M. C. B. V. de Souza, E. S. Lima and V. F. Ferreira, MedChemComm, 2010, 1, 229–232 RSC.
- M. K. Deliomeroglu, C. Dengiz, R. Caliskan and M. Balci, Tetrahedron, 2012, 68(29), 5838–5844 CrossRef CAS PubMed.
- M. Yilmaz, M. Yakut and A. T. Pekel, Synth. Commun., 2008, 38, 914–927 CrossRef CAS.
- G. Sartori, F. Bigi, G. Canali, R. Maggi, G. Casnati and X. Tao, J. Org. Chem., 1993, 58(4), 840–843 CrossRef CAS.
- F. Ameer Ameer, R. G. F. Giles, I. R. Green and R. Pearce, Synth. Commun., 2004, 34(7), 1247–1258 CrossRef PubMed.
- (a) N. Sperber, D. Papa, E. Schwenk and M. Sherlock, J. Am. Chem. Soc., 1949, 71, 887–889 CrossRef CAS; (b) K. Maruyama, T. Otsuki, K. Mitsui and M. Tojo, J. Heterocycl. Chem., 1980, 17, 695–700 CrossRef CAS PubMed; (c) F. Berthiol, H. Doucet and M. Santelli, Eur. J. Org. Chem., 2003, 6, 1091–1096 CrossRef PubMed; (d) K. Itami, T. Nokami, Y. Ishimura, K. Mitsudo, T. Kamei and J. Yoshida, J. Am. Chem. Soc., 2001, 123, 11577–11585 CrossRef CAS PubMed.
- (a) N. Lozac'h and J. Teste, C. R. Chim., 1952, 234, 1891–1893 CAS; (b) W. Skuballa, E. Schillinger, C.-S. Stürzebecher and H. Vorbrüggen, J. Med. Chem., 1986, 29, 315–317 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 883886. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c3ra48015a |
| This journal is © The Royal Society of Chemistry 2014 |