A rhodamine based “off-on” probe for selective detection of Hg(II) and subsequent L-proline and 4-hydroxyproline discrimination†
Ajoy Pal and
Bamaprasad Bag*
Colloids and Materials Chemistry Department, Academy of Scientific and Innovative Research, CSIR-Institute of Minerals and Materials Technology, P.O.: R.R.L., Bhubaneswar-751013, India. E-mail: bpbag@immt.res.in; Tel: +91 674 2379254
First published on 31st January 2014
Abstract
A substituted rhodamine based bifunctional chemosensor 2 showed “turn-on” chromogenic and fluorogenic dual mode signal modulation upon selective binding with Hg(II) whereas its Hg(II) complex selectively detects L-proline among all biologically significant amino acids by quenching of its enhanced fluorescence. In the process of ‘Off-On-Off’ signaling, the complex therefore discriminates L-proline and its hydroxyl derivative, which is a vital phenomenon of inducing selectivity among amino acid identification.
Metals, either in neutral or ionic form, are considered as a major constituent of global pollutants. Extracted and mining from the earth's crust, they are being used in various industries e.g. as fungicides, seed preservatives, etc. in agriculture, in pharmaceuticals, as pulp and paper preservatives, as catalysts in organic syntheses, in thermometers and batteries, in amalgams and in chlorine and caustic soda production. A few metals, present in certain quantities, are also essential in physiological perspective to perform various biological processes, such as functioning of critical enzymatic reactions and protein synthesis. There are a few heavy metals, called xenobiotics (e.g. lead, mercury), which hardly have any role in human physiology but are toxic1 even at trace level of exposure. The U.S. Agency for Toxic Substances and Disease Registry (ATSDR) has ranked Hg(II) ion in second position according to its prevalence and severity of their toxicity. As an environment pollutant, mercury dispersion through atmospheric deposition has increased markedly through waste ignition; interestingly, the medical industry is one of the largest contributor2 to mercury pollution. Exposure to high levels of metallic, inorganic, or organic form of mercury lead to severe health hazards3 in human being such as permanent damage to brain, kidneys, liver and developing fetus.3d Therefore development of highly selective and sensitive sensors for detection of Hg(II) ion in trace level is essential for environmental monitoring and human health perspective. Other modes of their detection4 have various drawbacks due to high expenses, sophisticated instrumentation, complicated sample preparation procedures, along with inherent sensitivity issues.
Molecular fluorescence sensors, due to their advantages in sensitivity, quick response time, non-evasive non-destructive nature and easy sample preparation, have proven to be an important tool for quantitative analysis of Hg(II) ion, particularly in molecular biology5 for studying details of metal toxicity and in environmental analysis. Many fluorescence sensors based on small molecules,6 short peptides,7 gold nanoparticle,8 QDs9 have been reported for detection trace amount of Hg(II) ion. On the other hand, chromogenic molecular probes offer a visual perception of photophysical changes for their detection as a function of colour change. Combining together, chemosensors with ability to undergo dual mode signaling are certainly advantageous in detection of metal ions. Chemosensors based on rhodamine dyes10 have already proven to be suitable in executing dual mode signaling action to detect various metal ions in trace quantities through their excellent yet contrasting structure–function correlation.
Further, detection of trace amount of amino acids is of enormous importance in diagnostic perspective. Commonly practiced analytical procedures like spectroscopic,11 chromatographic12 or electrochemical approaches13 for amino acid detection have several limitations. Recent advances14 in amino acid sensing have focused on usage of fluorescent and colorimetric methods for their selective and discriminative detection, owing to methodological and mechanistic advantages. In this context, only few xanthene dye based chemosensors have been reported15 for detection of various amino acids. Such an amino acid L-proline (L-pro), a primary constituent of collagen and many other proteins,16 is used as osmoprotectant in many pharmaceutical, biotechnological applications and as organo-catalyst, as precursor to many reactions, etc., and hence, its identification and selective detection is highly desired. Few QD based electro-chemiluminescence probes17 has been reported for L-pro detection. Although a fluorescence chemosensor15c for detection of N-Boc-protected proline is known, no chemosensors incorporating rhodamine has been reported so far for selective detection of L-pro. As discussed, rhodamine based probes provide synthetic, methodological and operational advantages in chemosensing applications, as well explore the possibility of detection of anions and neutral molecules using probe metal ion complex in a displacement approach.15b,18 We report herein the photo-physical signal modulation in a rhodamine B based bifunctional chemosensor 2 (Scheme 1), which not only exhibited selectivity in detection of Hg(II) ions in aqueous medium, but also its Hg(II) complex efficiently responded towards selective detection of L-pro in presence of various amino acids. Importantly, 2–Hg(II) complex is capable of discriminating L-pro in presence of its hydroxyl derivative 4-hydroxy proline (Hyp), which find a greater prospective as analytical method in various biological and industrial applications.
Condensation of aminoethyl-rhodamine 1 (ref. 19) with 2-pyridine carboxaldehyde in EtOH followed by reduction with NaBH4 afforded the reduced Schiff base compound 2 (see ESI†), which was characterized (ESI) through 1H-NMR, 13C-NMR and ESI-MS spectroscopic techniques. The colorless solution of probe 2 in MeCN–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, tris–HCl, pH 7.2) solution (conc. = 1 × 10−4 M) did not show any observable absorption in 500–700 nm region inferring to spirolactam conformation of rhodamine, which was also confirmed by appearance of the characteristic peak at ∼65 ppm in 13C-NMR spectra in CDCl3 (66.27 in CD3CN–D2O, 1
1 v/v, tris–HCl, pH 7.2) solution (conc. = 1 × 10−4 M) did not show any observable absorption in 500–700 nm region inferring to spirolactam conformation of rhodamine, which was also confirmed by appearance of the characteristic peak at ∼65 ppm in 13C-NMR spectra in CDCl3 (66.27 in CD3CN–D2O, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v).
1 v/v).
To investigate photo-physical spectroscopic changes of probe 2 in presence of various metal ions, aqueous solutions of metal perchlorate salts were added to its solution (MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O, 1
H2O, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
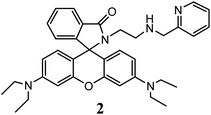
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, tris–HCl buffer, pH 7.2), then subsequent absorption and steady state fluorescence spectra were recorded. Probe 2 did not show any enhancement in either absorption or emission intensity on addition of Li(I), Na(I), K(I), Ca(II), Ba(II), Mn(II), Mg(II), Co(II), Ni(II), Zn(II), Cd(II) ions. However, when Hg(II) was added to the colorless solution of 2, it turned pink in colour along with a high absorption around 557 nm and fluorescence enhancement due to Hg(II) ion induced spirolactam ring opening (Fig. 1). The coordination of Hg(II) with 2 promotes its spirolactam ring opening in MeCN-aqueous medium to result in observable and appreciable spectroscopic changes; which is in good agreement with other substituted amino-ethyl-rhodamine based19b,20 probes. The competitive parameters between probe–metal interactions against hydration of metal ion determine the coordination module, in turn, spectroscopic signaling pattern here. In case of metal ions except Hg(II), an aqueous environment in the solvent preferably lead to their hydration over their complexation with 2, while Hg(II) favorably coordinates to 2 in comparison to its hydration and facilitates ring opening of rhodamine to induce photo-physical changes.
1 v/v, tris–HCl buffer, pH 7.2), then subsequent absorption and steady state fluorescence spectra were recorded. Probe 2 did not show any enhancement in either absorption or emission intensity on addition of Li(I), Na(I), K(I), Ca(II), Ba(II), Mn(II), Mg(II), Co(II), Ni(II), Zn(II), Cd(II) ions. However, when Hg(II) was added to the colorless solution of 2, it turned pink in colour along with a high absorption around 557 nm and fluorescence enhancement due to Hg(II) ion induced spirolactam ring opening (Fig. 1). The coordination of Hg(II) with 2 promotes its spirolactam ring opening in MeCN-aqueous medium to result in observable and appreciable spectroscopic changes; which is in good agreement with other substituted amino-ethyl-rhodamine based19b,20 probes. The competitive parameters between probe–metal interactions against hydration of metal ion determine the coordination module, in turn, spectroscopic signaling pattern here. In case of metal ions except Hg(II), an aqueous environment in the solvent preferably lead to their hydration over their complexation with 2, while Hg(II) favorably coordinates to 2 in comparison to its hydration and facilitates ring opening of rhodamine to induce photo-physical changes.
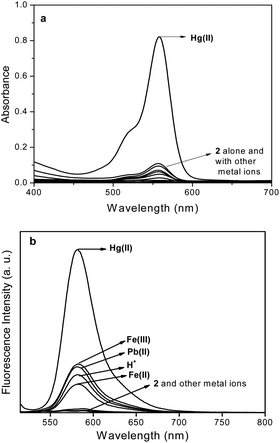 | ||
Fig. 1 Absorption (a) and fluorescence (b) spectra of 2 upon addition of various metal ions (10 eq.) or H+ in MeCN–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). Conc. [2] = 1 × 10−4 M (abs), 1 × 10−6 M (em), λex = 500 nm, RT. 1, v/v). Conc. [2] = 1 × 10−4 M (abs), 1 × 10−6 M (em), λex = 500 nm, RT. | ||
The observed increase in absorption (40–50 fold) transition of 2 around 557 nm on addition of few other metal ions such as Cr(III), Fe(III), Fe(II), Cu(II) and Pb(II) is much less in comparison to that of Hg(II) ion(∼500 fold), which promote the rhodamine spirolactam ring to open and turning the colour of the solution from colourless to pink rendering a chromogenic signal selectively and sensitively in it with Hg(II) ion. Fluorescence emission intensity of 2 significantly increased (∼120 fold) up on addition of Hg(II) as compare to that on addition of Cr(III), Fe(III), Fe(II), Cu(II) and Pb(II) ions (20–30 fold, Fig. 1b). Using continuous variation method (Job's plot), the stoichiometry of the complexes were determined to be in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (2
1 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) M(II)) ratio. The fluorescence titration pattern of 2 with Hg(II) ion lead to a sigmoidal curve (Fig. 2), exhibiting an increasing trend of fluorescence intensity with increase in concentration of Hg(II) ion up to 3 eq. and remained constant thereafter. The detection limit of probe 2 for Hg(II) was calculated to be 3.43 × 10−8 M, which is in consistent with that of other ‘aminoethyl rhodamine’ based19b,c,20 probes. From fluorescence titration, the binding constant was calculated to 4.24 × 108 M−1 for 2–Hg(II) complex by using Benesi–Hildebrand method for 1
M(II)) ratio. The fluorescence titration pattern of 2 with Hg(II) ion lead to a sigmoidal curve (Fig. 2), exhibiting an increasing trend of fluorescence intensity with increase in concentration of Hg(II) ion up to 3 eq. and remained constant thereafter. The detection limit of probe 2 for Hg(II) was calculated to be 3.43 × 10−8 M, which is in consistent with that of other ‘aminoethyl rhodamine’ based19b,c,20 probes. From fluorescence titration, the binding constant was calculated to 4.24 × 108 M−1 for 2–Hg(II) complex by using Benesi–Hildebrand method for 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexation.
1 complexation.
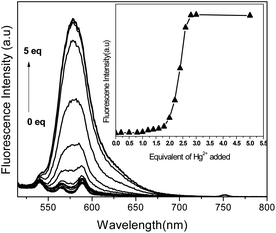 | ||
Fig. 2 Fluorescence titration spectra of 2 in MeCN–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) (conc. 2 = 1 × 10−6 M, λex = 500 nm, RT) with Hg(II) ion. 1 v/v) (conc. 2 = 1 × 10−6 M, λex = 500 nm, RT) with Hg(II) ion. | ||
Possible interferences by other metal ions were also assessed through competitive coordinating experiments with 2 in MeCN–H2O binary mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, tris–HCl, pH 7.2). The changes in its fluorescence intensity were measured after addition of 10 equivalents of Hg(II) followed by subsequent addition of other interfering metal ions (up to 100 eq.), which inferred that the fluorescence enhancement in 2 due to Hg(II) ion remains unaffected upon later addition of other competitive metal ion solutions. On contrary, the fluorescence of 2 which remains almost unaffected in presence of these competitive metal ion, gets enhanced on subsequent addition of Hg(II) up to the extent comparable to that of 2 in presence of Hg(II) alone. This establishes the probe's affinity towards Hg(II) ion over other competitive metal ions under similar experimental conditions.
1 v/v, tris–HCl, pH 7.2). The changes in its fluorescence intensity were measured after addition of 10 equivalents of Hg(II) followed by subsequent addition of other interfering metal ions (up to 100 eq.), which inferred that the fluorescence enhancement in 2 due to Hg(II) ion remains unaffected upon later addition of other competitive metal ion solutions. On contrary, the fluorescence of 2 which remains almost unaffected in presence of these competitive metal ion, gets enhanced on subsequent addition of Hg(II) up to the extent comparable to that of 2 in presence of Hg(II) alone. This establishes the probe's affinity towards Hg(II) ion over other competitive metal ions under similar experimental conditions.
When ammonium salts of various anions (F−, Cl−, Br−, NO3−, SO42−, CO32−, PO43−, Cr2O72−, AcO−) were added to pink coloured solution of 2–Hg(II) complex, most anions failed to induce any appreciable change in absorption or emission spectral pattern of spirolactam ring opened 2–Hg(II) complex (Fig. 3a) except F−, CO32− and AcO−, which significantly reduced absorbance and emission in the order of AcO− > CO32− > F−. The absorption and emission spectral pattern of delactonized rhodamine in 2–Hg(II) complex were observed to be unaltered upon addition of a competitive chelating ligand EDTA (ethylenediamine tetra acetic acid) but addition of En (ethylenediamine) reduced its spectral transition almost up to an extent of metal free 2. Thus, binding of Hg(II) with 2, which has shown reversibility in its signaling pattern with AcO− and ethylenediamine (Fig. 3), fulfills one of the essential requirement to be a molecular chemosensor.
 | ||
Fig. 3 (a) Absorbance spectra of 2–Hg(II) with different anions and (b) reversibility of 2–Hg(II) with ethylenediamine and AcO− ion(MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O 1 H2O 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v, [2] = 1 × 10−4 M). 1 v/v, [2] = 1 × 10−4 M). | ||
The reversible absorption and emission signaling pattern of 2 upon selective coordination to Hg(II) also promote to explore possibility of detection of amino acid exploiting their higher tendency to form complexes with metal ions. The solution of various amino acids (up to 20 eq.) were added to the pink coloured solution containing 2 and Hg(II) ion(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Hg(II)) and their photo-physical changes were investigated. From absorption and fluorescence spectral pattern of in situ 2–Hg(II) complex, it was observed that addition of L-pro rendered considerable decrease in absorption (Fig. 4) and emission (Fig. S7, ESI†) intensity while other amino acids failed to induced any such change. The pink colour of the solution also turned almost colourless upon addition of L-pro. Emission intensity (I582) of 2–Hg(II) solution (Fig. S7†) quenches up to an extent that of guest free 2, inferring to dislocation of Hg(II) ion in the probe–metal complex in favour of formation of stronger complex of Hg(II) with L-pro. Nevertheless, the absorption and fluorescence spectral pattern exhibited selectivity in L-pro detection among all the amino acids under investigation added to its solution (Fig. 5).
Hg(II)) and their photo-physical changes were investigated. From absorption and fluorescence spectral pattern of in situ 2–Hg(II) complex, it was observed that addition of L-pro rendered considerable decrease in absorption (Fig. 4) and emission (Fig. S7, ESI†) intensity while other amino acids failed to induced any such change. The pink colour of the solution also turned almost colourless upon addition of L-pro. Emission intensity (I582) of 2–Hg(II) solution (Fig. S7†) quenches up to an extent that of guest free 2, inferring to dislocation of Hg(II) ion in the probe–metal complex in favour of formation of stronger complex of Hg(II) with L-pro. Nevertheless, the absorption and fluorescence spectral pattern exhibited selectivity in L-pro detection among all the amino acids under investigation added to its solution (Fig. 5).
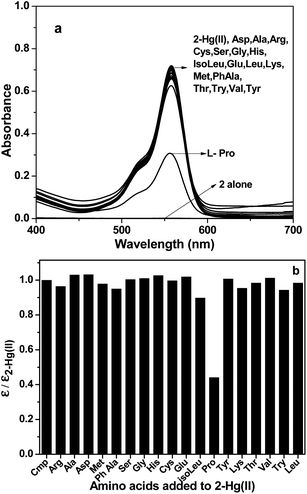 | ||
Fig. 4 Absorption spectra (a) and corresponding bar diagram (b) of 2–Hg(II) upon addition of (100 eq.) different amino acids in MeCN–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v). 1 v/v). | ||
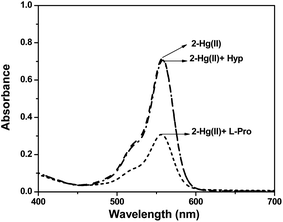 | ||
Fig. 5 Absorption spectra of 2–Hg(II) upon addition of L-pro and Hyp in MeCN–H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v), [2] = 1 × 10−4 M. 1 v/v), [2] = 1 × 10−4 M. | ||
When Hyp, mostly occurring substituted L-pro, was added to pink coloured solution of in situ formed 2–Hg(II), the colour and its absorption/fluorescence intensity were observed to remain unaffected. Although crystallographic structural evidences on complexes of L-pro/Hyp with Hg(II) is not apparent, correlation to thermodynamic parameters of their complexes21 with other 3d-metal ions infers that 2–Hg(II) complex rendered favorable spatial disposition and orientation for effective L-pro coordination with Hg(II). The steric hindrance due to presence of an hydroxyl group and conformational22 variation possibly also restricted Hyp coordination with Hg(II) ion and thus, fails to induce optical spectroscopic changes to that of 2–Hg(II) complex. The different spectral behavior of the complex observed towards L-pro and Hyp in solution thus able to discriminate between these two structurally-symmetrical biologically important amino acids.
In summary, the rhodamine B derivatized probe 2 exhibited high selectivity in coordination preference with Hg(II) ions over coexisting metal ions to result in ‘turn-on’ signaling pattern. The absorption and fluorescence intensity of its Hg(II) complex quenched along with a colour transition from pink to colourless upon addition of only L-pro among different amino acids investigated. Further, the contrast photophysical signaling behavior of 2–Hg(II) complex in presence of L-pro and Hyp allows it to discriminate between these two derivatives. Such induction of selectivity in signaling with metal–probe complex should encourage methodological design of chemosensory probes for selective and quantitative detection of various amino acids and other small molecules of biological and environmental interest. We are presently working along this direction.
Acknowledgements
BPB wishes to thank to DST, New Delhi for financial support (SB/EMEQ-226/2013) and research fellowship to AP; to the Director, CSIR-IMMT for infrastructural support.Notes and references
- (a) W. F. Fitzgerald, C. H. Lamborg and C. R. Hammerschmidt, Chem. Rev., 2007, 107, 641 CrossRef CAS PubMed; (b) O. Malm, Environ. Res., 1998, 77, 73 CrossRef CAS PubMed; (c) T. W. Clarkson, Crit. Rev. Clin. Lab. Sci., 1997, 34, 369 CrossRef CAS PubMed; (d) M. Harada, Crit. Rev. Toxicol., 1995, 25, 1 CrossRef CAS PubMed.
- J. Harvie, Public Health Rep., 1999, 114, 353 CrossRef CAS PubMed.
- (a) I. Oniyido, A. R. Norris and E. Buncel, Chem. Rev., 2004, 104, 5911 CrossRef PubMed; (b) H. H. Harris, I. J. Pickering and G. N. George, Science, 2003, 301, 1203 CrossRef CAS PubMed; (c) D. W. Boening, Chemosphere, 2000, 40, 1335 CrossRef CAS; (d) Y. Zhang, L. Gao, L. Wen, L. Heng and Y. Song, Phys. Chem. Chem. Phys., 2013, 15, 11943 RSC.
- (a) O. T. Butler, J. M. Cook, C. F. Harrington, S. J. Hill, J. Rieuwerts and D. L. J. Miles, J. Anal. At. Spectrom., 2006, 21, 217 RSC; (b) Y. Li, C. Chen, B. Li, J. Sun, J. Wang, Y. Gao, Y. Zhao and Z. J. Chai, J. Anal. At. Spectrom., 2006, 21, 94 RSC; (c) M. Leermakers, W. Baeyens, P. Quevauviller and M. Horvat, Trends Anal. Chem., 2005, 24, 383 CrossRef CAS PubMed.
- M. Taki, K. Akaoka, S. Iyoshi and Y. Yamamoto, Inorg. Chem., 2012, 51, 13075 CrossRef CAS PubMed.
- Y. Wang, Y. Huang, B. Li, L. Zhang, H. Song, H. Jiang and J. Gao, RSC Adv., 2011, 1, 1294 RSC.
- E. Pazos, O. Vazquez, J. L. Mascarenas and M. E. Vazquez, Chem. Soc. Rev., 2009, 38, 3348 RSC.
- (a) C.-Y. Lin, C.-J. Yu, Y.-H. Lin and W.-L. Tseng, Anal. Chem., 2010, 82, 6830 CrossRef CAS PubMed; (b) D. Tan, Y. He, X. Xing, Y. Zhao, H. Tang and D. Pang, Talanta, 2013, 113, 26 CrossRef CAS PubMed; (c) G. Sener, L. Uzun and A. Denizli, Anal. Chem., 2014, 86, 514 CrossRef CAS PubMed.
- (a) B. Liu, F. Zeng, G. Wu and S. Wu, Analyst, 2012, 137, 3717 RSC; (b) D. Huang, C. Niu, X. Wang, X. Lv and G. Zeng, Anal. Chem., 2013, 85, 1164 CrossRef CAS PubMed.
- Few recent reviews: (a) X. Chen, T. Pradhan, F. Wang, J. S. Kim and J. Yoon, Chem. Rev., 2012, 112, 1910 CrossRef CAS PubMed; (b) M. J. Culzoni, A. M. Pena, A. Machuca, H. C. Goicoechea and R. Babiano, Anal. Methods, 2013, 5, 30 RSC; (c) M. Beija, C. A. M. Afonso and J. M. G. Martinho, Chem. Soc. Rev., 2009, 38, 2410 RSC; (d) H. N. Kim, M. H. Lee, H. J. Kim, J. S. Kim and J. Yoon, Chem. Soc. Rev., 2008, 37, 1465 RSC.
- C. S. Lee, P. F. Teng, W. L. Wong, H. L. Kwong and A. S. C. Chan, Tetrahedron, 2005, 61, 7924 CrossRef CAS PubMed.
- J. Wang, M. Chtrathi and B. Tian, Anal. Chem., 2000, 72, 5774 CrossRef CAS.
- D. Vardanega and C. Giradet, Chem. Phys. Lett., 2009, 469, 172 CrossRef CAS PubMed.
- Y. Zhou and J. Yoon, Chem. Soc. Rev., 2012, 41, 52 RSC.
- (a) H. Wang, G. Zhou, H. Gai and X. Chen, Chem. Commun., 2012, 48, 8341 RSC; (b) L. Xu, Y. Xu, W. Zhu, B. Zeng, C. Yang, B. Wu and X. Qian, Org. Biomol. Chem., 2011, 9, 8284 RSC; (c) Z. Xing, Y. Fu, J. Zhou, C. Zhu and Y. Cheng, Org. Biomol. Chem., 2012, 10, 4024 RSC.
- (a) J.-W. Handgraaf and F. Zerbetto, Proteins, 2006, 64, 711 CrossRef CAS PubMed; (b) C. L. Jenkins, L. E. Bretscher, I. A. Guzei and R. T. Raines, J. Am. Chem. Soc., 2003, 125, 6422 CrossRef CAS PubMed.
- (a) H. Hosono, W. Satoh, J. Fukuda and H. Suzuki, Sens. Actuators, B, 2007, 122, 542 CrossRef CAS PubMed; (b) M. Zhang, F. Wan, S. Wang, S. Ge, M. Yan and J. Yu, J. Lumin., 2012, 132, 938 CrossRef CAS PubMed.
- (a) X. Lou, L. Zhang, J. Qin and Z. Li, Langmuir, 2010, 26, 1566 CrossRef CAS PubMed; (b) C. Luo, Q. Zhou, B. Zhang and X. Wang, New J. Chem., 2011, 35, 45 RSC; (c) Y.-B. Ruan, A.-F. Li, J.-S. Zhao, J.-S. Shen and Y.-B. Jiang, Chem. Commun., 2010, 46, 4938 RSC.
- (a) X. Zhang, Y. Shiraishi and T. Hirai, Org. Lett., 2007, 9, 5039 CrossRef CAS PubMed; (b) B. Bag and A. Pal, Org. Biomol. Chem., 2011, 9, 4467 RSC; (c) B. Bag and B. Biswal, Org. Biomol. Chem., 2012, 10, 2733 RSC.
- A. Pal and B. Bag, J. Photochem. Photobiol., A, 2012, 240, 42 CrossRef CAS PubMed.
- S.-G. Park, Y.-J. Kim, S.-N. Choi and Y.-I. Kim, Bull. Korean Chem. Soc., 2001, 22, 779 CAS.
- A. E. Aliev and D. Courtier-Murias, J. Phys. Chem. B, 2007, 111, 14034 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Absorption and fluorescence spectral measurements, synthetic procedure of probes and spectroscopic characterization. See DOI: 10.1039/c3ra48013e |
| This journal is © The Royal Society of Chemistry 2014 |