An on-chip RT-PCR microfluidic device, that integrates mRNA extraction, cDNA synthesis, and gene amplification
Nari Hana,
Jeong Hwan Shinb and
Ki-Ho Han*a
aDepartment of Nano Science and Engineering, Center for Nano Manufacturing, Inje University, 607 Obang-dong, Gimhae 621-749, Gyongnam, Republic of Korea. E-mail: mems@inje.ac.kr; Fax: +82-55-320-3631; Tel: +82-55-320-3715
bDepartment of Laboratory Medicine, Busan Paik Hospital, College of Medicine, Inje University, Busan 614-735, Republic of Korea
First published on 20th January 2014
Abstract
This paper presents an on-chip integrated reverse transcriptase polymerase chain reaction (RT-PCR) microchip, which integrates the genetic functionalities of messenger ribonucleic acid (mRNA) extraction, complementary deoxyribonucleic acid (cDNA) synthesis, and gene amplification. The proposed RT-PCR microchip consists of a RNA microchannel for extracting mRNA from lysate biological samples, and a DNA microchamber for synthesizing cDNA and amplifying the target gene. As magnetic oligo-dT beads are passed through the RNA microchannel, mRNA bound to the beads was extracted within 1 min by a ferromagnetic wire array inlaid in the RNA microchannel. In the DNA microchamber, the extracted bound mRNA was used for direct synthesis of cDNA followed by amplification of the target gene. To evaluate the performance of the RT-PCR microchip, RT-PCR analyses were performed using various volumes of blood and numbers of breast cancer cells. Additionally, to verify the feasibility of the RT-PCR microchip for clinical applications, it was used for detection of virus-specific genes from specimens of patients infected with respiratory viruses. The continuous and integrated mRNA extraction, cDNA synthesis, and gene amplification process enabled by the proposed RT-PCR microchip suggests its use for high-precision genetic assays with small sample quantities.
1. Introduction
Reverse transcription polymerase chain reaction (RT-PCR) is a common technique used to quantify specific ribonucleic acid (RNA) molecules within cells or tissues, which can be applied in molecular biology,1,2 genetic disease diagnosis,3–5 and forensics.6,7 The extraction of high-quality messenger RNA (mRNA) from biological samples is critical for accurate and efficient RT-PCR performance. mRNA is destroyed readily by ribonucleases (RNases) from bacteria and molds present on human skin, as well as from dust in the environment. Because the ubiquitous RNases are highly active, it is difficult to purify high-quality mRNA without contamination and degradation.Due to the necessity for time-consuming sample preparation with conventional RT-PCR methods, RNA degradation by RNases is difficult to avoid. Thus, performing an accurate RT-PCR assay using small quantities of biological samples is challenging. Closed, RNase-free, rapid RNA extraction is essential to avoid RNA degradation and so achieve high-precision RT-PCR analysis. Microfluidic devices8–15 with a closed, integrated format to have been developed prevent RNase contamination and achieve high-quality genetic assays. Due to their high sensitivity, some RT-PCR microfluidic devices16–19 can perform genetic assays using a single cell. Although the previous reported RT-PCR microfluidic devices have been successfully demonstrated their performance, the majority of the RT-PCR microfluidic devices did not include the RNA purification functionality or used the solid phase extraction method for extracting nucleic acids, which contain DNA as well as RNA. It may causes that they have the limited accuracy.
Previously, our group reported a reverse transcription (RT)-microchip,20 which consists of a RNA microchannel and a complementary deoxyribonucleic acid (cDNA) synthesis microchamber. In the RNA microchannel, mRNA was extracted within 1 min by lateral magnetophoresis with magnetic oligo-dT beads,21 cDNA was then immediately synthesized in the cDNA microchamber. Our results showed that the RT-microchip had a higher sensitivity than conventional RT-PCR methods. In this case, gene amplification was performed outside the device separately, using cDNA harvested from the RT-microchip.
As an upgraded version of the previously reported RT-microchip, this paper introduces a fully integrated RT-PCR microchip, which includes mRNA extraction, cDNA synthesis, and gene amplification. The microchip was composed of polydimethylsiloxane (PDMS) and glass. To prevent leakage of the reagent in the DNA microchamber during thermal cycling for gene amplification, a thermo-hardening epoxy was injected through the waste outlet to create a fluid-tight seal between the RNA microchannel and the DNA microchamber. To extract mRNA from a lysate sample, lateral magnetophoresis22 and magnetic oligo-dT beads23 were used. A typical hotplate and a commercialized thermal cycler were utilized for cDNA synthesis and gene amplification, respectively. The performance of the RT-PCR microchip was evaluated with respect to the volume of whole blood and the number of breast cancer cells used. Additionally, to verify its usefulness for clinical applications, the RT-PCR microchip was used to detect influenza virus genes in specimens from patients infected with parainfluenza, rhinovirus A, respiratory syncytial virus, and metapneumovirus.
2. Experimental
2.1 Working principle
Consider a ferromagnetic wire array placed at an angle θ with respect to the direction of flow, and inlaid over the entire area of the RNA microchannel (Fig. 1). When an external magnetic field is laterally applied to the RNA microchannel, the inlaid ferromagnetic wire generates a high-gradient magnetic field (HGMS)24,25 over the entire RNA microchannel. Thus, magnetic oligo-dT beads passing over the wire experience a magnetic force Fm with a hydrodynamic drag force Fd, as shown in Fig. 1. The lateral magnetic force, Fl, on a bead is the vector sum of the magnetic force and the drag force. With an external magnetic field, the magnetic beads with bound mRNA are therefore forced laterally, and flow into the DNA microchamber, whereas residue from in the lysate sample flows into the waste outlet (Fig. 1).The separated magnetic beads are collected in the DNA microchamber by a permanent magnet. For cDNA synthesis and gene amplification using the mRNA bound to the beads, a reagent mixture for cDNA synthesis and gene amplification is injected into the DNA microchamber through the reagent inlet. The magnetic beads with bound mRNA are retained within the DNA microchamber. After mixture injection, the RT-PCR microchip is placed on a hotplate and cDNA is synthesized from the mRNA. Immediately following cDNA synthesis, the target gene is amplified using a commercial PCR thermocycler. Therefore, mRNA extraction, cDNA synthesis, and gene amplification, are performed continuously within the RT-PCR microchip.
2.2 Design
The proposed RT-PCR microchip consists of the RNA microchannel and the DNA microchamber. The RNA microchannel includes inlaid ferromagnetic wires (permalloy; Ni0.8Fe0.2) used to extract mRNA with the magnetic oligo-dT beads. The DNA microchamber synthesizes cDNA and amplifies the target gene. The microchip includes three inlets (for the sample, buffer, and reagent) and two outlets (for the DNA and waste), as shown in Fig. 1. Two syringe pumps (Legato 200, KD Scientific, Inc.) were used to inject a lysate sample mixed with magnetic beads and buffer solution through the sample and buffer inlets. The flow rates of the lysate sample and buffer were both 15 mL h−1, which induces a laminar flow in the RNA microchannel. Laminar flow is crucial for high-quality mRNA extraction, because it produces a multistream fluid in which the magnetic beads with bound mRNA are selectively transported across the lysate sample and buffer stream, before flowing into the DNA microchamber.The length, width, and height of the RNA microchannel were 30 mm, 1 mm, and 70 μm, respectively. To enrich the magnetic oligo-dT beads, the width of the channel connected to the cDNA microchamber was 200 μm; i.e., one-quarter that of the channel connected to the waste outlet (800 μm). The width and thickness of the inlaid ferromagnetic wire were 50 and 20 μm, respectively. For the RNA microchannel to contain six ferromagnetic wires, the angle, θ, between the wire and the direction of flow was 5.7°, and the distance between the wires was 300 μm. Due to the strong magnetic force, some magnetic beads stacked at the corners between the ferromagnetic wires and the sidewall of the RNA microchannel, thereby getting lost in the microchannel; this reduced the mRNA extraction rate significantly. To prevent this, the ferromagnetic wire was bent 84.3° at 100 μm distal from the sidewall of the RNA microchannel. As a result, a portion of the ferromagnetic wire behind the bend point was aligned parallel the external magnetic field. When the ferromagnetic wire and the external magnetic field are parallel, no magnetic field gradient develops, preventing the stacking of magnetic oligo-dT beads in the corners.26
The DNA microchamber was designed to have a streamlined shape to avoid trapping air bubbles. To contain 40 μL of the reagent mixture, including 20 μL of cDNA synthesis reagents and 20 μL of PCR reagents, the length, width, and height of the DNA microchamber were 10, 4, and 1 mm, respectively.
2.3 Fabrication process
The microfabrication process for the RT-PCR microchip used glass slides of 0.7 mm thickness (Borofloat33 Pyrex, Schott AG) and a PDMS mold as the primary construction materials. To form the inlaid ferromagnetic wires, a Ti/Cu/Cr seed layer (200/2000/1000 Å) for permalloy (Ni0.8Fe0.2) electroplating was electron-beam evaporated onto a glass substrate. Photoresist (AZ9260; AZ Electronic Materials) was spun and patterned to create micromolds for 50 μm thick ferromagnetic wires (Fig. 2(a)). Ferromagnetic wires 40 μm in thickness were electroplated onto the glass substrate. After removing the photoresist, epoxy adhesive (HE #200; Hyundae Epoxy Chemicals Co.) was poured onto the substrate and cured for 6 h at 85 °C to form a flat surface. Consequently, a 20 μm thick ferromagnetic wire was formed and inlaid on the glass substrate by mechanical polishing (Fig. 2(b)). A 2 μm thick PDMS layer was spun onto the glass substrate, and cured for 60 min at 85 °C.An SU-8 mold on a glass master was fabricated for the PDMA replica, which included the RNA microchannel (70 μm height) for mRNA extraction and a DNA microchamber (1 mm height) for cDNA synthesis and gene amplification. A 70 μm thick layer of SU-8 2050 photoresist (MicroChem Corp.) was spun and patterned, creating a mold for the RNA microchannel on the glass master, along with an evaporated 2000 Å Cr layer to increase the adhesion between SU-8 and the glass master. Epoxy-based dry film sheets (SUEX; DJ DevCorp), of 1 mm thickness, were used to fabricate the DNA microchamber mold on the glass master, including the RNA microchannel SU-8 mold. The polymer mold, fabricated by stereolithography (Viper SI2, 3D Systems), was used to pour the liquid PDMS. The PDMS mold was completed by assembling the glass master and polymer mold. The liquid-phase PDMS, produced by mixing the resin and curing agent in a 10 to 1 ratio (Sylgard 184, Dow Corning), was poured into the PDMS mold and cured for 60 min at 85 °C in an oven (Fig. 2(c)). After peeling the PDMS replica off of the PDMS mold, the inlet and outlet reservoirs of the PDMS replica were generated using a 1.5 mm diameter punch (Fig. 2(d)).
The glass substrate with the inlaid ferromagnetic wire array and the PDMS replica were treated with oxygen plasma for 60 s at 6.8 W RF power (PDC-32G-2, Harrick Plasma). They were then aligned and bonded using a PDMS aligner (MDA-4000B, Midas System) (Fig. 2(e)). Fig. 3(a) shows the fabricated RT-PCR microchip.
3. Materials and methods
3.1 Preparation of the magnetic oligo-dT beads
To extract mRNA, we used 2.8 μm diameter magnetic beads (Dynabeads® Oligo(dT)25, Invitrogen) to bind oligo-dT sequences. A solution containing the magnetic beads was first suspended in a vial by agitation to obtain a uniform brown suspension. Then, 100 μL of the solution, as a suspension containing 3.7 × 104 beads per μL, was transferred to a 1.5 mL tube. The beads were washed twice with lysis/binding buffer (Dynabeads mRNA Direct kit, Invitrogen), and placed on ice.3.2 Preparation of lysate samples
3.3 mRNA extraction
A tube containing the washed magnetic oligo-dT beads was placed on a magnet for 1 min to collect the beads, and the solution was discarded. To bind mRNA to the magnetic beads, the prepared lysate sample was added to the magnetic beads and mixed by pipetting for 5 min at room temperature. The lysate bead mixture was then injected at a flow rate of 15 mL h−1 by two syringe pumps. Two 3 mL plastic syringes (BD Biosciences) were connected to the two inlets by capillary tubing (Teflon® FER 1/16 in. tubing, 0.25 mm i.d., IDEK). Tris–EDTA was used as the buffer solution for the whole blood and cell line samples, and washing buffer A (Dynabeads mRNA Direct kit) for the clinical specimens. A neodymium–iron–boron (Nd–Fe–B) permanent magnet was placed at the side of the RT-PCR microchip, generating an external magnetic flux of ∼0.14 T, which was applied in a lateral direction with respect to the RNA microchannel. The RT-PCR microchip was placed under a microscope (ME600; Nikon Instruments Inc.) to enable monitoring of the magnetic beads passing over the inlaid ferromagnetic wire array, as shown in Fig. 3(b) and (c).3.4 cDNA synthesis and gene amplification
For gene amplification, a commercially available thermocycler (GeneAmp PCR System 9700, Applied Biosystems) was used. Standard PCR thermocycling was performed using following procedure: 94 °C for 5 min, followed by 40 cycles of 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s, followed by a final extension at 72 °C for 10 min. The sequences of the forward and reverse primers used to amplify a 244-bp fragment of the human β-actin gene were 5′-GTACCACTGGCATCGTGATGGA-3′ and 5′-GCCATCTCTTGCTCGAAGTCCAG-3′, respectively. A 211-bp fragment of the KRT19 cancer-specific gene was amplified using the primer pairs 5′-TTTGAGACGGAACAGGCTCT-3′ and 5′-AATCCACCTCCACACTGACC-3′. Due to the pressure caused by the high temperatures used for gene amplification, the reagent mixture could potentially leak from the DNA microchamber. Thus, to prevent leakage, a thermo-hardening epoxy (Flow-Mix 5 Minute Epoxy, Devcon) was injected through the waste outlet, thereby achieving fluid-tight sealing between the RNA microchannel and the DNA microchamber.
After gene amplification, the amplified gene in the DNA microchamber was harvested through the DNA outlet. The gene was then resolved in a 2% agarose gel (Molecular Biology Grade, Invitrogen) with ethidium bromide (Promega) staining, followed by observation under ultraviolet (UV) light. Product sizes were estimated by comparison with fluorescently labeled DNA standards, and processed using the ImageJ software (NIH).
4. Results and discussion
4.1 Whole blood
To verify the performance of the proposed RT-PCR microchip, a human housekeeping gene (β-actin, 244 bp) was amplified from whole blood lysate. Fig. 4(a) shows that the human β-actin gene was successfully detected by the RT-PCR microchip using 100 to 0.1 μL blood. As expected, the fluorescence intensity of the agarose gel band decreased with the volume of blood used. The ImageJ software was used to quantify the fluorescence intensity for a specific volume of blood to evaluate the performance of the RT-PCR microchip. The mean fluorescence intensity of three measurements varied from 15 to 1, as the volume of blood varied from 100 to 0.1 μL (Fig. 4(b)). Therefore, the proposed RT-PCR microchip can be used to detect the human β-actin gene in 0.1 μL of blood.4.2 Breast cancer cell lines
To quantify the sensitivity of the RT-PCR microchip, the cancer-specific gene keratin19 (KRT19, 211 bp) was amplified from 105 to 1 SKBR3 breast cancer cells. The fluorescence intensity of the agarose gel band decreased with the number of cancer cells (Fig. 5(a)). Using the ImageJ software, the fluorescence intensity was quantified to evaluate the RT-PCR microchip. The mean fluorescence intensity of three measurements decreased from 15 to 0.5 as the number of cancer cells decreased from 105 to 1 (Fig. 5(b)). Therefore, single-cell RT-PCR analysis is possible using the proposed RT-PCR microchip. mRNA was extracted rapidly from the lysate sample using the RT-PCR microchip, thereby reducing RNase contamination and so facilitating high-sensitivity RT-PCR analysis.4.3 Clinical specimens
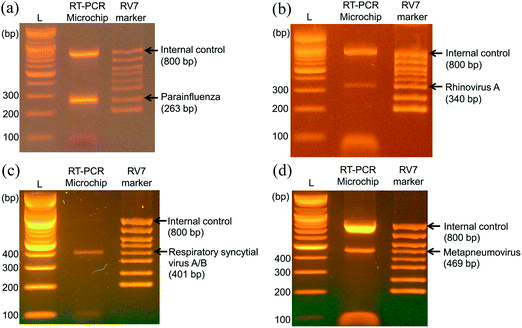
To evaluate the feasibility of the RT-PCR microchip for clinical applications, RT-PCR analysis of specimens infected with influenza virus was performed. A specific gene (263 bp) was identified in the specimen of a patient infected with parainfluenza virus, which occurs mainly in children less than 5 years of age (Fig. 6(a)). Additionally, other influenza virus genes (340, 401, and 469 bp) were respectively measured in the specimens of patients infected with rhinovirus A (Fig. 6(b)), respiratory syncytial virus (Fig. 6(c)), and metapneumovirus (Fig. 6(d)). Because the number of infected cells in each specimen varied, the measured gene expression levels also differed. Therefore, the proposed RT-PCR microchip successfully detected respiratory influenza viruses in clinical specimens. Taken together, these evaluations of the performance demonstrated the high-precision and wide range of applications of the proposed RT-PCR microchip.5. Conclusions
We developed an on-chip integrated RT-PCR microchip in which mRNA extraction, cDNA synthesis, and gene amplification were integrated. By the lateral magnetophoretic technique with magnetic oligo-dT beads, mRNA from lysate samples was extracted within 1 min. Due to the integrated nature of the RT-PCR microchip, the number of processing steps was minimized compared with conventional RT-PCR methods that require pipetting and the use of a number of tubes. The biological sample in the RT-PCR microchip is completely isolated from the outside environment, preventing RNase contamination. The fact that use of an extremely small quantity of sample, such as 0.1 μL of whole blood or a single cell, is possible suggests the use of the proposed RT-PCR microchip for high-precision genetic analysis. Additionally, the microchip protects researchers from exposure to viral infection due to its closed configuration. Therefore, it is safe for use in clinical applications. Because the RT-PCR microchip has the advantages of a simple structure and on-chip integrated format, beginners as well as highly trained individuals can conduct accurate RT-PCR assays. Moreover, if developed to include an automatic control system, the proposed RT-PCR microchip could contribute to the global standardization of RT-PCR assays.Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST) (Grant no. NRF-2011-0008248 and NRF-2012R1A2A2A03045174).References
- L. Lin, L. Chamberlain, L. J. Zhu and M. R. Green, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 1997–2002 CrossRef CAS PubMed.
- T. D. Schmittgen, B. A. Zakrajsek, A. G. Mills, V. Gorn, M. J. Singer and M. W. Reed, Anal. Biochem., 2000, 285, 194–204 CrossRef CAS PubMed.
- R. J. Torres, M. G. Garcia and J. G. Puig, Gene, 2012, 511, 306–307 CrossRef CAS PubMed.
- C.-S. Liao, G.-B. Lee, H.-S. Liu, T.-M. Hsieh and C.-H. Luo, Nucleic Acids Res., 2005, 33, e156 CrossRef PubMed.
- T. Takano, A. Miyauchi, T. Yokozawa, F. Matsuzuka, G. Liu, T. Higashiyama, S. Morita, K. Kuma and N. Amino, Cancer Res., 1998, 58, 4913–4917 CAS.
- M. Setzer, J. Juusola and J. Ballantyne, J. Forensic Sci., 2008, 53, 296–305 CrossRef CAS PubMed.
- J. Juusola and J. Ballantyne, Forensic Sci. Int., 2003, 135, 85–96 CrossRef CAS.
- D. C. Saunders, G. L. Holst, C. R. Phaneuf, N. Pak, M. Marchese, N. Sondej, M. McKinnon and C. R. Forest, Biosens. Bioelectron., 2013, 44, 222–228 CrossRef PubMed.
- K. J. Shaw, E. M. Hughes, C. E. Dyer, J. Greenman and S. J. Haswell, Lab. Invest., 2013, 93, 961–966 CrossRef CAS PubMed.
- Q. Cao, M. Mahalanabis, J. Chang, B. Carey, C. Hsieh, A. Stanley, C. A. Odell, P. Mitchell, J. Feldman, N. R. Pollock and C. M. Klapperich, PLoS One, 2012, 7, e33176 CAS.
- K. A. Hagan, C. R. Reedy, M. L. Uchimoto, D. Basu, D. A. Engel and J. P. Landers, Lab Chip, 2011, 11, 957–961 RSC.
- M. Ritzi-Lehnert, R. Himmelreich, H. Attig, J. Claußen, R. Dahlke, G. Großhauser, E. Holzer, M. Jeziorski, E. Schaeffer, A. Wende, S. Werner, J. O. Wiborg, I. Wick, K. S. Drese and T. Rothmann, Biomed. Microdevices, 2011, 13, 819–827 CrossRef CAS PubMed.
- N. Beyor, L. Yi, T. S. Seo and R. A. Mathies, Anal. Chem., 2009, 81, 3523–3528 CrossRef CAS PubMed.
- K.-Y. Lien, J.-L. Lin, C.-Y. Liu, H.-Y. Lei and G.-B. Lee, Lab Chip, 2007, 7, 868–875 RSC.
- J. S. Marcus, W. F. Anderson and S. R. Quake, Anal. Chem., 2006, 78, 956–958 CrossRef CAS PubMed.
- D. J. Eastburn, A. Sciambi and A. R. Abate, Anal. Chem., 2013, 85, 8016–8021 CrossRef CAS PubMed.
- S. Furutani, H. Nagai, Y. Takamura, Y. Aoyama and I. Kubo, Analyst, 2012, 137, 2951–2957 RSC.
- A. K. White, M. VanInsberghe, O. I. Petriv, M. Hamidi, D. Sikorski, M. A. Marra, J. Piret, S. Aparicio and C. L. Hansen, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 13999–14004 CrossRef CAS PubMed.
- N. M. Toriello, E. S. Douglas, N. Thaitrong, S. C. Hsiao, M. B. Francis, C. R. Bertozzi and R. A. Mathies, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 20175–20178 CrossRef PubMed.
- H. Lee, N. Han, I.-H. Choi and K.-H. Han, Biomed. Microdevices, 2013, 15, 9–15 CrossRef CAS PubMed.
- H. Lee, J. Jung, S.-I. Han and K.-H. Han, Lab Chip, 2010, 10, 2764–2770 RSC.
- J. Jung and K.-H. Han, Appl. Phys. Lett., 2008, 93, 223902 CrossRef PubMed.
- G. Fonnum, C. Johansson, A. Molteberg, S. Mørup and E. Aksnes, J. Magn. Magn. Mater., 2005, 293, 41–47 CrossRef CAS PubMed.
- K.-H. Han and A. B. Frazier, J. Appl. Phys., 2004, 96, 5797–5802 CrossRef CAS PubMed.
- D. Melville, F. Paul and S. Roath, IEEE Trans. Magn., 1975, 11, 1701–1704 CrossRef.
- S. Kim, S.-I. Han, M.-J. Park, C.-W. Jeon, Y.-D. Joo, I.-H. Choi and K.-H. Han, Anal. Chem., 2013, 85, 2779–2786 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2014 |