A simple route to synthesize hierarchical porous ZrO2†
Yu Chen*a,
Hui Xiaa,
Daiguo Zhanga,
Zilai Yanb,
Fangping Ouyangb,
Xiang Xiongb and
Xiaoyi Huang*c
aHunan Key Laboratory for Super-microstructure and Ultrafast Process, Central South University, 932 Lushan Nan Rd, Changsha, Hunan 410083, P.R. China. E-mail: chenyu_8323@csu.edu.cn
bPowder Metallurgy Research Institute, State Key Laboratory of Powder Metallurgy, 932 Lushan Nan Rd, Changsha, Hunan 410083, P.R. China
cPhysics Department, Xiangnan University, Chenzhou, Hunan 423000, P.R. China. E-mail: jghxy@126.com
First published on 14th January 2014
Abstract
A simple method for preparing porous ZrO2 has been proposed by directly decomposing a Zr(NO3)4·5H2O ethanol sol–gel solution. Contrary to other traditional sol–gel methods, porous ZrO2 with multi-sized macro- and meso-pores are synthesized via only one simple step. The size of the macropores ranges from 100 nm to 500 nm. Remarkably, we also isolated some highly dispersed ZrO2 nanoparticles (∼60 nm), which on their surface or even in the whole body are fully filled with well-sized ever-smaller spherical mesopores with an average diameter around ∼5 nm. A further investigation of low-temperature nitrogen absorption has shown a high surface area over 162 m2 g−1 for the fully crystallized ZrO2. No more additional modifiers or complicated preparations are needed in the present method, which promises a new potential option in fabricating high-porosity materials.
Hierarchical porous metal oxides on multiple scales have attracted considerable attention due to their interesting physical and chemical properties.1,2 These types of materials often exhibit high specific surface area, good permeability and low relative density, promising potential application in sensors, catalyzers as well as gas purification.3–6 Thus, any approaches that could achieve these materials efficiently and inexpensively are of great interests. Up to now, many typical technologies – such as those based on hard or soft templates,7,8 self-assembly processes with a help of specific surfactants,3,9,10 etc. – have been developed to meet this requirement. However, traditional approaches above need either additional modifiers or complicated pre-preparation and post-treating processes. For example, template-based methods need to build a porous framework first, then insert cared materials into the template, and finally remove the pre-created template away if necessary. This is a complicated project and even in its first step, to a large extent, is sometimes a difficult and costly task. In addition, removing the pore-forming frameworks is another big challenge. In contrast, self-assembly processes are comparatively simple. But the method usually requires specific surfactants or pore-forming agents. These surfactants and agents are expensive and how to remove them at the end of the fabrication is also a big problem.
ZrO2 is one of the most important ceramic materials, which is widely used in oxygen sensors, fuel cells, catalysts and catalyst supports.11–13 These applications can be attributed to its unique physical properties. It is the only traditional metal oxide having both acid and base active centers on its surface, which shows excellent catalytic activity on some processes of the alkylation and oxidation.4,14,15 ZrO2, as a widely used catalyst or air sensor, requires high specific surface area and strong adsorptive capacity. Increasing the porosity of ZrO2 or decreasing its particle size is a candidate option. However different from SiO2, TiO2, reports on the preparing and manufacturing of porous ZrO2 are very limited. Fujita et al. successfully prepared a porous crystalline zirconia by combining a sol–gel and solvothermal process.3 They achieved a high surface area precursor of ZrO2 gels. However, as mentioned by Fujita et al. themselves, the collapse of microstructures in the high temperature of sintering (>400 °C) results in a following great loss of surface area. Hong et al. fabricated a ZrO2 porous ceramic using a camphene-based freeze-casting method.6 But the method requires some comparatively special preparation conditions and procedures.
Here, we proposed a new and simple method with which hierarchical porous ZrO2 was fabricated via only one decomposing step of Zr(NO3)4·5H2O ethanol sol–gel solution. In the decomposition products, we found some micron-sized ZrO2 blocks that full filled with spherical air pores. After a further separation process, we also isolated some of highly dispersed ZrO2 nanoparticles (∼60 nm), which on their surface or even in the whole body are full of well-sized ever-smaller spherical mesopores with the average diameter around ∼5 nm. The successful fabrication of these porous ZrO2 suggests a new optional approach for preparing high-porosity metal oxides.
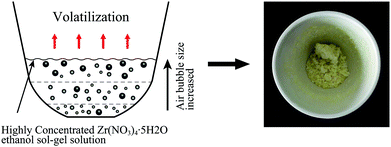
The entire fabrication process is described as illustrated in Fig. 1(a). The concentration of the sol–gel precursor, chemical reactions to generate porous ZrO2 gels and the final annealing process were all finished in the same alumina crucible. The new method is, to a large extent, able to avoid the introduction of unwanted impurities. Furthermore, in order to fabricate a porous ZrO2, air bubbles emitted in the thermal decomposition process (NO2 bubbles) were utilized as a soft template. This kind of soft template also has been successfully applied in the fabricating of porous hierarchical In2O3.16 The biggest advantage of using air bubbles as a template is that it requires no further treatments at the end of the synthesis. The precursor, however, is another important factor which provides a growth-environment for both air bubbles and ZrO2. This requires a liquid of high concentration, low flowability and a fast gelation process accompany with abundant air emission. But the solubility of Zr(NO3)4·5H2O powder in ethanol at room temperature is much lower than this requirement, and there is no significant changes even at the boiling point of this solution. But we found if we firstly prepared a diluent solution, and then let it being volatilized slowly at 60 °C, it will be able to form a transparent gel–sol solution with a very high concentration. Thus in this article, 10 g Zr(NO3)4·5H2O powder was firstly mixed with 30 mL of anhydrous ethanol to form a relatively diluent solution. Then the solution was heated up to 60 °C just a little lower than its boiling point and volatilizing at this temperature slowly to achieve a high concentration. As we know, the volatilizing process is usually very slow. Thus we volatilized at 60 °C is for the purpose to accelerate this step. The heating rate is well controlled at a very slow speed because present Zr(NO3)4 sol–gel solution is easily to be hydrolyzed directly at a high-speed of heating or if the temperature is higher than 80 °C. The reaction process is depicted in Fig. 1(a). As the precursor has been concentrated to a very high level, creating a liquid of poor flowability, air bubbles (with a small size) in this circumstance will be jammed and aggregated where it was created. These air bubbles are emitted simultaneously and utilized as a soft template in the gelation when the temperature reached 90 °C. Fig. 1(b) shows the synthesized amorphous ZrO2 gels.
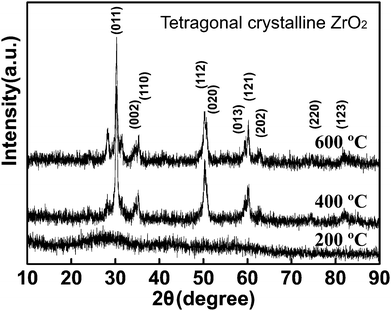
The achieved ZrO2 gels were dried in the air for another 2 hours and then transferred to muffle furnace annealing at different temperature. Fig. 2 is the XRD results and shows a tetragonal crystalline ZrO2 if the sintering temperature is higher than 400 °C.
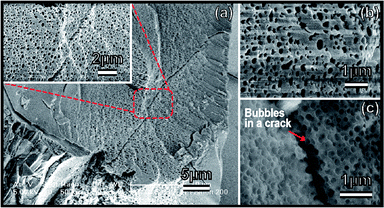
The morphologies and microstructures of the achieved ZrO2 after crystallization were carefully investigated by FESEM and the results are shown in Fig. 3. A large area of macro-pores on a micron-sized ZrO2 block can be clearly observed. It is not a cross-linked air channel as reported in the ref. 3, but regular spherical air bubbles on the surface or embedded inside of the ZrO2. Generally, polymerization-induced bicontinuous pores (in ref. 3) are easily to be collapsed sintering in high-temperature. However, air bubbles utilized as pore-forming template can be well preserved in present new method (see in Fig. 3). From FESEM images of Fig. 3(c), we can infer that these spherical air pores are not only existed on the materials' surface, but also inside of the ZrO2. The size distribution estimated from Fig. 3(b) is ranged from 100 nm to 500 nm. It should be pointed out that this bubble-embedded ZrO2 is not the only product obtained in the reaction. There are other reactants that have distinct micro-structures (please see the details in the ESI†). In other works, we will focus on the factors, such as pressure, solution concentration, etc., that could determine the formation of pores.
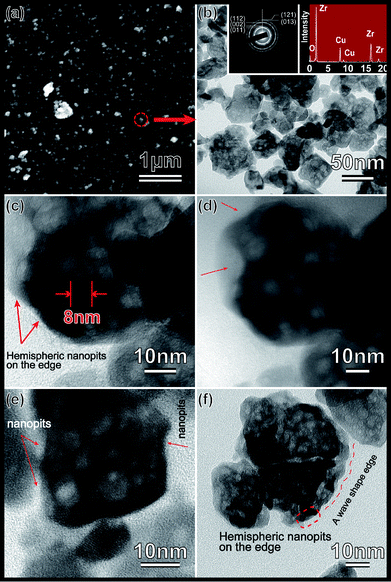
The achieved ZrO2 in the last step was further pulverized into powder by an agate mortar. Remarkably, we isolated some of mesoporous ZrO2 particles this time. The powder was ultrasonic oscillated in anhydrous ethanol for another 1 hour and then left untouched until a stable and homogeneous suspension was obtained. As we know that pure ethanol has a very low surface tension. Only those materials with high surface area can be suspended stably in this solution. Thus we can infer that the isolated ZrO2 particles possess a considerable high surface area. A further investigation of the low-temperature nitrogen absorption has confirmed these conclusions which shows a high BET surface area of over 162 m2 g−1. Fig. 5(a) shows the nitrogen adsorption–desorption isotherm curve. This kind of isotherm indicates the existence of closed pores. A copper grid is then put into the ZrO2 suspension and lift up for the next SEM and TEM investigations to further examine this assumption. The detailed SEM and FETEM analysis results are shown in Fig. 4. Fig. 4(a) shows SEM images of the highly dispersed ZrO2 nanoparticles. Red circle selected area was a possible region that will be further characterized using FETEM. The FETEM results are presented in Fig. 4(b)–(f). Nanopores which are spherical and with average diameter around ∼5 nm were founded spreading on the surface of a comparative bigger ZrO2 seeds (∼60 nm). Pore size distribution obtained from nitrogen absorption data shows that it is centered at 50 Å. In the present experiment, the decomposing process is simultaneously along with the gelation process which emits abundant red-brown NO2 gases. The emitted NO2 will form a mass of bubbles in the liquid by the action of surface tension. However, because the liquid at this moment is concentrated to a very high level and the phase transformation is finished so fast that NO2 bubbles, which have not enough time to be grown up and moved out of the liquid, are frozen and aggregated where they were created and finally to be utilized as a soft pore-forming template. The color of achieved ZrO2 gels (as show in Fig. 1(b)) are of red-brown at the beginning and beige some hours latter. This phenomenon indicates that ZrO2 gels have absorbed a considerable amount of NO2 gases in the gelation. However, after dried in air for some hours, the absorbed NO2 have finally been released.
From the results in Fig. 4 and 5, we were still unable to confirm whether there are air bubbles or pores embedded inside of these isolated ZrO2 nanoparticles. But what we can make sure is that hemispheric nanopits are full filled on their surface. Fig. 4(d) is a lower magnification FETEM image for the same particle investigated in Fig. 4(c) which can gives a full view of the particle edge. Red arrows in these two figures marked clearly the signs of hemispheric nanopits because of its irregular edge surface. Fig. 4(e) and (f) is the image of another two distinct ZrO2 nanoparticles. It is indicated that the phenomena of edge surface irregularity is not a single case, but widely existed in these isolated ZrO2 nanoparticles.
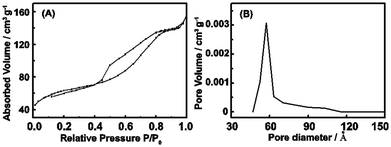 | ||
| Fig. 5 Nitrogen adsorption characterizations of the isolated porous ZrO2 powder. (a) Nitrogen adsorption–desorption isotherms. (b) The pore size distribution data. | ||
Only Zr, O and Cu (from Cu grid) were detected in the energy dispersive spectrum (EDS) tests (see Fig. 4(a)), which confirmed the chemical elements. The electron diffraction pattern (see Fig. 4(a)) matches well with the tetragonal ZrO2 phase, which the diffraction rings {011}, {002}, {112}, {013} and {121} has been indexed.
In summary, a novel and simple approach for preparing hierarchical porous ZrO2 has been proposed using air bubbles as a template. ZrO2 with multi-sized macro- and meso-pores can be easily synthesized via directly decomposing highly concentrated Zr(NO3)4·5H2O ethanol sol–gel solution at 90 °C. Air bubbles originated from this fast decomposing process were simultaneously utilized as the soft template. The bulk ZrO2 material fabricated using this template is full filled with spherical air bubbles and, this bubble framework can still be well preserved even after a high temperature of annealing. Macro-pores analyzed by SEM are ranged from 100 nm to 500 nm. Remarkably, we also isolated some of highly dispersed ZrO2 nanoparticles (∼60 nm) which on their surface or even in the whole body are distributed meso-pores with average diameter around ∼5 nm. No more additional modifiers or complicated preparations are needed in the present method, which promises a new potential option in fabricating porous ZrO2.
Methods
Hierarchical porous ZrO2 are synthesized by directly decomposing highly concentrated ZrO2 gel–sol solution. The precursor was prepared through dissolving Zr(NO3)4·5H2O in ethanol. The used regents of Zr(NO3)4·5H2O and alcohol were analytic grade, purchased from Sinopharm Chemical Reagent Shanghai Co., Ltd. Firstly, 10 g Zr(NO3)4·5H2O powder was mixed with 30 mL of anhydrous ethanol at room temperature. Because of its poor solubility, the Zr(NO3)4 suspending liquid was heated up to 40 °C and stirred until it was completely dissolved. Then in the second step of volatilization, the temperature was raised to 60 °C and highly concentrated, transparent ZrO2 gel–sol solution was achieved after about 1 hour of volatilization. Finally, when ZrO2 gel–sol solution started to get viscous (total concentration is more than 90%), the temperature was heated up quickly to 90 °C. Along with this progress, a large amount of red-brown gas was released and fluffy ZrO2 (see Fig. 1(b)) filled with hierarchical pores was obtained. After drying two hours in the air, ZrO2 zirconia was then transferred into muffle furnace for high temperature annealing.The chemical equation is as following:
| 3Zr(NO3)4·5H2O + CH3CH2OH → 3ZrO2 + 18H2O + 2CO2↑ + 12NO2↑ |
The synthesized ZrO2 after annealing at different temperatures were examined by X-ray diffraction (XRD) measurements on a D/max-2550/PC instrument with a scanning speed of 2 degrees per minute (see Fig. 2). FESEM (filed emission scanning electron microscope) tests were operated on an FEI Sirion 200 with an acceleration voltage of 5.0 kV. HRTEM (high-resolved transmission electron microscopy) measurements were taken on a JEM-2100f instrument under an acceleration voltage of 200 kV. Energy dispersive X-ray spectra (EDS) were also utilized to confirm the chemical components of the samples. The size distribution of the pore size was calculated from the nitrogen adsorption data using the Barrett–Joyner–Halenda (BJH) method, and the surface are was obtained by the Brunauer–Emmett–Teller (BET) method.
Acknowledgements
This work is financially supported by the National Science Foundation of China the (no. 51002009), the Fundamental Research Funds for the Central Universities (no. 2012QNZT055) and Hunan provincial Natural Science Foundation of China (no. 13jj3005). We greatly appreciate the support from Instrumental Analysis Center of Shanghai Jiao Tong University, Shanghai, P. R. China.References
- G. J. Zhang, J. F. Yang and T. Ohji, Fabrication of Porous Ceramics with Unidirectionally Aligned Continuous Pores, J. Am. Ceram. Soc., 2001, 84, 1395–1397 CrossRef CAS.
- Z. Y. Yuan and B. L. Su, Insights into Hierarchically Meso/Macroporous Structured Materials, J. Mater. Chem., 2006, 16, 663–667 RSC.
- J. Konishi, K. Fujita, S. Oiwa, K. Nakanishi and K. Hirao, Crystalline ZrO2 Monoliths with Well-Defined Macropores and Mesostructured Skeletons Prepared by Combining the Alkoxy-Derived Sol–Gel Process Accompanied by Phase Separation and the Solvothermal Process, Chem. Mater., 2008, 20, 2165–2173 CrossRef CAS.
- S. Benfer and E. Knözinger, Structure, morphology and surface properties of nanostructured ZrO2 particles, J. Mater. Chem., 1999, 9, 1203–1209 RSC.
- J. Zhou and C.-A. Wang, Porous Yttria-Stabilized Zirconia Ceramics Fabricated by Nonaqueous-Based Gelcasting Process with PMMA Microsphere as Pore-Forming Agent, J. Am. Ceram. Soc., 2013, 96, 266–271 CrossRef CAS.
- J. Han, C. Hong, X. Zhang, J. Du and W. Zhang, Highly porous ZrO2 ceramics fabricated by a camphene-based freeze-casting route: Microstructure and properties, J. Eur. Ceram. Soc., 2010, 30, 53–60 CrossRef CAS PubMed.
- M.-L. Wang, C.-H. Wang and W. Wang, Preparation of porous ZrO2/Al2O3 macrobeads from ion-exchange resin templates, J. Mater. Sci., 2011, 46, 1220–1227 CrossRef CAS.
- H. Ma, Y. Kong, W. Hou and Q. Yan, Synthesis of Ordered Hexagonal Porous Tin-Doped Zirconium Oxides with a High Surface Area, Microporous Mesoporous Mater., 2005, 77, 241–243 CrossRef CAS PubMed.
- J. Blin, A. Leonard, Z. Yuan, L. Gigot, A. Vantomme, A. Cheetham and B. Su, Hierarchically Mesoporous/Macroporous Metal Oxides Templated from Polyethylene Oxide Surfactant Assemblies, Angew. Chem., Int. Ed., 2003, 42, 2872 CrossRef CAS PubMed.
- G. Pacheco, E. Zhao, A. Garcia, A. Sklyarov and J. J. Fripiat, Syntheses of mesoporous zirconia with anionic surfactants, J. Mater. Chem., 1998, 8, 219–226 RSC.
- Y. Chen, J. Gu, D. Zhang, S. Zhu, H. Su, X. Hu, C. Feng, W. Zhang, Q. Liua and A. R. Parker, Tunable three-dimensional ZrO2 photonic crystals replicated from single butterfly wing scales, J. Mater. Chem., 2011, 21, 15237–15243 RSC.
- J. F. Haw, J. Zhang, K. Shimizu, T. N. Venkatraman, D.-P. Luigi, W. Song, D. H. Barich and J. B. Nicholas, NMR and Theoretical Study of Acidity Probes on Sulfated Zirconia Catalysts, J. Am. Chem. Soc., 2000, 122, 12561 CrossRef CAS.
- I. J. Dijs, J. W. Geus and L. W. Jenneskens, Effect of Size and Extent of Sulfation of Bulk and Silica-Supported ZrO2 on Catalytic Activity in Gas- and Liquid-Phase Reactions, J. Phys. Chem. B, 2003, 107, 13403–13413 CrossRef CAS.
- T. Yamagushi, Application of ZrO2 as a catalyst and a catalyst support, Catal. Today, 1994, 20, 199 CrossRef.
- R. Takahashi, S. Sato, T. Sodesawa, K. Suzuki, M. Tafu, K. Nakanishi and N. Soga, Phase Separation in Sol–Gel Process of Alkoxide-Derived Silica–Zirconia in the Presence of Polyethylene Oxide, J. Am. Ceram. Soc., 2001, 84, 1968–1976 CrossRef CAS.
- L. Gai, L. Ma, H. Jiang, Y. Ma, Y. Tian and H. Liu, Nitrogen-doped In2O3 nanocrystals constituting hierarchical structures with enhanced gas-sensing properties, CrystEngComm, 2012, 14, 7479–7486 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra47967f |
| This journal is © The Royal Society of Chemistry 2014 |