Controlling the doping level of double-walled carbon nanotubes by using aromatic hydrocarbon complexes†
Kyoung-Yong Chun*
Center for Bio-Artificial Muscle and Department of Biomedical Engineering, Hanyang University, Seoul, 133-791, South Korea. E-mail: kychun@gmail.com; Fax: +82 2 2291 2320; Tel: +82 2 2220 4540
First published on 17th January 2014
Abstract
The level of potassium doping in double-walled carbon nanotubes is controlled by using the potassium–aromatic hydrocarbon (AH) complexes. The concentration of doping increase when the large AH molecule complex is delivered. The crystalline perfection is also affected by the degree of K-doping.
Carbon nanotubes (CNTs) have been investigated for many applications such as nanocomposites, supercapacitors, energy harvesting and solar cells. The adjustment of optic, electronic, and the mechanical properties of CNTs have required the morphological and structural modification of CNTs.1–4
For instance, doping into CNTs using potassium (K), nitrogen, boron, and cesium is a reliable method to modify the surface of CNTs and their electrical properties.5–10 Alkali metal doping such as K has particularly attracted much attention on the modification of the electronic properties of CNTs.11–13 Some research groups reported the enhancement of electrical and thermal conductivity of CNTs, control of the K-doping level by reversibly intercalating and deintercalating, and short channel n-type CNT field-effect transistors with K-doped source and drain regions.14–16 K-doping into CNTs has been conventionally performed using the reaction of vaporized potassium in a complex procedure with high-energy consumption and a high cost process.15
Previously, we demonstrated the effective chemical K-doping in double-walled CNTs (DWNTs) at room temperature and its characterizations.17 Recently, Li et al. reported in vitro detection of superoxide anions released from cancer cells based on K-doped CNT-ionic liquid composite gels18 and the nitrite-selective sensing using K-modified graphene by using chemical K-doping at room temperature.19 However, the facile control of K-doping level has not been released yet.
In this communication, the effect of aromatic hydrocarbons (AHs) on the determination of K-doping level and crystalline perfection of the DWNTs were demonstrated, which are chemically doped at room temperature.
High-quality DWNTs were synthesized by arc discharge (AD) method. The as-synthesized DWNTs have ∼3.5 nm in diameter. The impurities of CNTs was removed by air oxidation at 450 °C for 1 h with conventional acid treatment.20 Chemical complexes for K-doping were prepared by reacting K (200 wt% to DWNTs) with 0.2 mol dm−3 of three AHs (benzene, naphthalene, and anthracene) in 20 ml of 1,2-dimethoxyethane (1,2-DME, 99.5%). Purified DWNTs (20 mg) were reacted with chemical complexes using bath sonication and then stirred for 48 h using magnetic bar with 500 rpm at room temperature. The resulting product was thoroughly washed several times with ethanol. The morphologies and structures of K-doped DWNTs were characterized by scanning electron microscopy (SEM) (Hitachi, S-4700) and high-resolution transmission electron microscopy (TEM) (TECNAI, F20, 200 kV). X-ray photoelectron spectroscopy (XPS) (VG-Scientific ESCALAB 250 spectrometer) was measured to confirm K-doping (X-ray: monocromatized Al Kα line, step energy: 1486.6 eV with 0.1 eV resolution). Thermal analysis of the DWNTs was conducted by thermogravimetric analysis (TGA) (TA instruments, Q-50, 5 °C min−1). Raman spectroscopy (Jobin–Yvon, LabRam HR) was operated using 514.5 nm Ar laser excitation to observe the defect degree of K-doped DWNTs.
To obtain the distribution of K atoms, XPS was measured using photon energy 1486.6 eV. The C at% against total components was relatively decreased forward to the long AHs (Fig. S1, ESI†). Interestingly, as shown in Fig. 1a, the concentration of doping increased from 5.8 to 7.7 at% with increase of the number of C6-ring in the AHs. In the case of anthracene complex, doping concentration showed highest value as 7.7 at%. In our previous work, the K-doping concentration in the DWNTs grown by chemical vapor deposition (CVD) is 3.2 at% when phenanthrene complex used.17 In general, CNTs synthesized by AD method have high crystallinity compared to CNTs grown by CVD method. In this study, the degree of K-doping of AD-DWNTs is larger than that of CVD-DWNTs even though the reason of different doping concentration has not been proved. The typical K 2p spectrum comprises two peak components at the binding energy of 293.3 and 296 eV.21 The difference of binding energy between these two peak components was about 2.7 eV and their intensity ratio was about 1.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Two binding energies are ascribed to the spin–orbit–split doublet (K 2p3/2 and K 2p1/2) of the K-oxides and K-cations, respectively.22 Here, no metallic K peak found corresponding to 294.6 eV.
1. Two binding energies are ascribed to the spin–orbit–split doublet (K 2p3/2 and K 2p1/2) of the K-oxides and K-cations, respectively.22 Here, no metallic K peak found corresponding to 294.6 eV.
In Fig. 1b, the C 1s peak for the K-doped DWNTs was observed at 284.6 eV and was very consistent with sp2 graphite carbon. The peak at 284.6 eV is assigned to the C–C and C–H bonding. As a number of C6-ring of AHs increased, the area of C 1s was degraded from 65.5 to 54.1 at%. Undoped DWNTs was 72.6 at%. It indicates that the C6-rings in the AH complexes affects the consistent reduction of C-atoms binding and the generation of disorder or defects in the DWNTs.
Usually, the K-doping using chemical reaction method is achieved by large expansion stress inside of DWCNT bundles entangled by strong van der Waals force. Actually, due to the swallow potential along the direction of K+ ion transfer in the isomer of K+–AHs, K+ cation easily moves over the AHs plane.23 In our system, the various AHs–K complexes give different intensity of stress to the CNTs and may intercalate the inside both of CNT bundles and graphite sheets, providing an each doping degree in the CNTs.
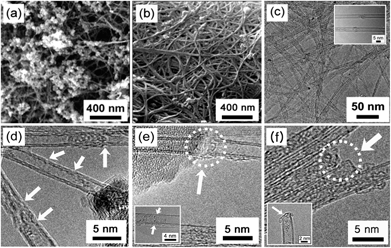
Fig. 2a shows the SEM image of as-synthesized CNTs including carbonaceous particles and many metal catalyst particles. After purification, the large amounts of entangled carbon filaments were observed (Fig. 2b). The K-doping was additionally confirmed by using energy dispersive spectrometer (EDS) in the case of benzene–K complexes (Fig. S2, ESI†). The distribution of K in the CNTs looks very broadly in the acceptable active area as 5.1 at% which is consistent with XPS analysis. The amorphous carbon and metallic catalyst were almost removed as shown in the TEM image of Fig. 2c. A highly ordered crystalline structure was also observed in the inset of Fig. 2c. After K-doping, the morphologies of DWNTs was changed to defective and disordered aspect as shown in Fig. 2d–f. Interestingly, the degree of defects tends to rise according to the increase of C6-ring in the AHs. In the case of anthracene with three C6-rings, more defects and cuttings in the CNTs were observed than those in other cases of AHs. Here, it is found that the types of K–AHs complexes afford not only the difference of K-doping concentration, but also the degree of defects in the DWNTs.
Theoretically, the binding energy between AHs and K+ ions is slightly decreased according to the increase of C6-ring in the AHs.23,24 In other words, the highly mobile K+ ions is more easily transported under large C6-ring complex and effectively doped in the DWNTs. In particular, anthracene, linear AHs with large size, can introduce a more consecutive binding energy than that of other AHs and is able to afford mapping on the large surface area of CNTs to form π-stacking interaction. Consequently, the difference of binding energies between AHs and K+ ions, which provide intercalation compounds, can be an important factor to achieve the difference of K-doping level. Moreover, the attack of K–AHs complexes to the surface of CNTs can give a large enlargement of bundles and layer spacing in the CNTs, resulting in the degradation of crystalline perfection. Regarding the AHs with DWNTs in the absence of potassium, the degradation of DWNTs can be occurred because the AH molecules easily slide and move on a C–C bond of the DWNT surface.25 In other words, complex solution mixtures without potassium are able to make the degradation of DWNT surfaces because the role of the AHs ion radicals is to guide and attack the doping reaction in the solution. However, the optimized-complex mixtures including potassium, AHs and solvent with different binding energy mainly contribute to give stress accompanied by the vacancies of CNT bundle networks and the enlargement of interlayer spacing of CNTs.17
TGA curves for each DWNTs were demonstrated (Fig. S3, ESI†). The decomposition was carried out in ambient air at a low heating rate of 5 °C min−1. The initial purity of the purified DWNTs is about 89%. It indicates that the residual impurities including iron and iron oxide catalyst are mostly eliminated. Interestingly, the gasification temperature shows a shift to the low temperature after K-doping, but purity is almost the same with pristine DWNTs. It is known that the defect or disorder of DWCNTs is increased and residual impurities are a little removed due to the K-doping.
Raman spectroscopy was also studied to confirm the change of crystalline perfection owing to the K-doping. The variations of the radial breathing mode (RBM) frequencies of the K-doped DWNTs are shown in Fig. 3a. As increasing C6-ring of AHs, the intensity of high frequencies in the RBM mode decreases. Also, the peaks indicating the DWNTs with small diameter disappear due to the structural deformation of the DWNTs after K-doping. The G band at 1592.6 cm−1 and the D band at 1344.4 cm−1 were detected in tangential mode (Fig. 3b). The G band correspond to stretching and bending E2g mode due to the in-plane vibrational movement of carbon atoms.26 The D band indicate A1g (vibrational mode) caused by disorder and defect of C–C bond, which is a highly dispersive spectral feature.27 The tangential mode shows no significant line shifts or broadening according to the K-doping concentration. The G band splits into two components G+ at 1592.6 and a G− at 1570.9 cm−1 due to the different force constants for C–C bonds along the tube length and circumferential directions.28 The significant changes of intensity and shift in the G+ and G− frequencies were not observed, suggesting that the force constant for the C–C bonds along the tube length and circumferential direction in bundled DWNTs is not largely affected by the intercalation of K–AHs complexes.
 | ||
| Fig. 3 Raman spectra using 514.5 nm Ar laser: (a) radial breathing modes (low frequency), (b) tangential modes (high frequency). | ||
However, the disorder-induced D band of tangential mode shows a consistent increase in intensity, which the slow incorporation of K atoms might result in the change of defect degree in the hexagonal lattice of DWNTs. Also, the reduction of the intensity value of crystallization (IG/ID), in the range from 10.3 to 7.9, implies that the graphitization of DWNTs is clearly degraded according to more active reaction with longer C6-ring in the AH complexes and CNTs. Therefore, the behavior of crystalline perfection of DWNTs is strongly attributed to the types of AHs complexes during the K-doping.
Conclusions
In conclusions, the control of potassium doping level of the DWNTs is possible by using the types of aromatic hydrocarbons–potassium complexes in appropriate polar solvent. The combination of aromatic hydrocarbons and potassium is very important factor to make different doping level in the DWNTs. Like aromatic hydrocarbons, the guidance of metal alkali can play an important role to tailor the mechanical and electrical properties of carbon nanotubes even though the precise control of chemical doping is further required.Notes and references
- K.-Y. Chun, Y. Oh, J. Rho, J.-H. Ahn, Y.-J. Kim, H. R. Choi and S. Baik, Nat. Nanotechnol., 2010, 5, 853 CrossRef CAS PubMed.
- Z. Tang, C. Tang and H. Gong, Adv. Funct. Mater., 2012, 22, 1272 CrossRef CAS.
- J. Lohrman, C. X. Zhang, W. Zhang and S. Q. Ren, Chem. Commun., 2012, 48, 8377 RSC.
- Y. Jo, J. Y. Cheon, J. Yu, H. Y. Jeong, C.-H. Han, Y. Jun and S. H. Joo, Chem. Commun., 2012, 48, 8057 RSC.
- R. S. Lee, H. J. Kim, J. E. Fischer, A. Thess and R. E. Smalley, Nature, 1997, 388, 255 CrossRef CAS PubMed.
- M. Terrones, Small, 2005, 1, 1032 CrossRef CAS PubMed.
- J. C. Carrero-Sańchez, A. L. Elías, R. Mancilla, G. Arrellín, H. Terrones, J. P. Laclette and M. Terrones, Nano Lett., 2006, 6, 1609 CrossRef PubMed.
- R. Sen, B. C. Satishkumar, A. Govindaraj, K. R. Harikumar, M. K. Renganathanb and C. N. R. Rao, J. Mater. Chem., 1997, 7, 2335 RSC.
- M. Nath, B. C. Satishkumar, A. Govindaraj, C. P. Vinod and C. N. R. Rao, Chem. Phys. Lett., 2000, 322, 333 CrossRef CAS.
- C.-L. Sun, H.-W. Wang, M. Hayashi, L.-C. Chen and K.-H. Chen, J. Am. Chem. Soc., 2006, 128, 8368 CrossRef CAS PubMed.
- L. Grigorian, G. U. Sumanasekera, A. L. Lpoer, S. Fang, J. L. Allen and P. C. Eklund, Phys. Rev. B: Condens. Matter Mater. Phys., 1998, 58, R4195 CrossRef CAS.
- G. Gao, T. Cagin and W. A. Goddard III, Phys. Rev. Lett., 1998, 80, 5556 CrossRef CAS.
- T. Miyake and S. Saito, Phys. Rev. B: Condens. Matter Mater. Phys., 2002, 65, 165419 CrossRef.
- A. M. Rao, P. C. Eklund, S. Bandow, A. Thess and R. E. Smalley, Nature, 1997, 388, 257 CrossRef CAS PubMed.
- M. Bockrath, J. Hone, A. Zettle, P. L. McEuen, A. G. Rinzler and R. E. Smalley, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 61, R10606 CrossRef CAS.
- A. Javey, R. Tu, D. B. Farmer, J. Guo, R. G. Gordon and H. Dai, Nano Lett., 2005, 5, 345 CrossRef CAS PubMed.
- K.-Y. Chun and C. J. Lee, J. Phys. Chem. C, 2008, 112, 4492 CAS.
- X.-R. Li, B. Wang, J.-J. Xu and H.-Y. Chen, Nanoscale, 2011, 3, 5026 RSC.
- X.-R. Li, F.-Y. Kong, J. Liu, T.-M. Liang, J.-Y. Xu and H.-Y. Chen, Adv. Funct. Mater., 2012, 22, 1981 CrossRef CAS.
- B. Haand and C. J. Lee, Appl. Phys. Lett., 2007, 90, 023108 CrossRef PubMed.
- H. H. Huang, X. Jiang, Z. Zou, W. S. Chin, G. Q. Xu, W. L. Dai, K. N. Fan and J. F. Deng, Surf. Sci., 1998, 412, 555 CrossRef.
- S. Li, E. T. Kang, K. G. Neoh, Z. H. Ma, K. L. Tan and W. Huang, Appl. Surf. Sci., 2001, 181, 201 CrossRef CAS.
- S. Ikuta, J. Mol. Struct.: THEOCHEM, 2000, 530, 201 CrossRef CAS.
- S. Hashimoto and S. Ikuta, J. Mol. Struct.: THEOCHEM, 1999, 468, 85 CrossRef CAS.
- F. Tournus and J. C. Charlier, Phys. Rev. B: Condens. Matter Mater. Phys., 2005, 71, 165421 CrossRef.
- C. Thomsen and S. Reich, Phys. Rev. Lett., 2000, 85, 5214 CrossRef CAS.
- N. R. Raravikar, P. Keblinski, A. M. Rao, M. S. Dresselhaus, L. S. Schadler and P. M. Ajayan, Phys. Rev. B: Condens. Matter Mater. Phys., 2002, 66, 235424 CrossRef.
- M. S. Dresselhaus and P. C. Eklund, Adv. Phys., 2000, 49, 705 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra47807f |
| This journal is © The Royal Society of Chemistry 2014 |