DOI:
10.1039/C3RA47780K
(Paper)
RSC Adv., 2014,
4, 15650-15654
Synthesis of zinc–nickel ferrite nanorods and their magnetic properties
Received
19th December 2013
, Accepted 18th March 2014
First published on 19th March 2014
Abstract
Zinc–nickel (Zn–Ni) ferrite nanorods were synthesized by a microemulsion-based method in combination with calcination at different temperatures. The morphologies and structures of Zn–Ni ferrite nanorods and their precursors of Zn–Ni–Fe composite oxalate nanorods were characterized using field emission scanning electron microscopy and X-ray diffraction. Magnetization measurements were carried out using a vibrating sample magnetometer at room temperature. It was shown that the Zn–Ni ferrite nanorods were around 50–200 nm in diameter and several micrometers in length. Their saturation magnetization increased with increasing calcination temperature from 350 to 900 °C. In addition, the magnetic properties of the Zn–Ni ferrite nanorods were also affected by their composition.
1. Introduction
Soft ferrites are commercially important materials on account of their electrical and magnetic properties.1 Spinel-type ferrites, with general formula AFe2O4, are widely used in many electronic and magnetic devices because of their high magnetic permeability and low magnetic loss.2 Among them, nickel ferrite (NiFe2O4) has been intensively investigated as a magnetic nanomaterial.3–7 Recently, doping of inverse spinel ferrites with zinc has been used to improve their electrical or magnetic properties.8–11 Zinc–nickel (Zn–Ni) ferrite is a versatile ferrite from the viewpoint of its large number of applications due to its high magnetic permeability, high electrical resistivity, high Curie temperature, and low power loss at high frequencies.12–14 At present, many routes are developed to prepare Zn–Ni ferrite nanoparticles such as thermal decomposition,13 inert gas condensation,14 co-precipitation,15 hydrothermal reaction,16 sol–gel process17,18 and ball milling.19 However, few reports involve the one-dimensional (1D) Zn–Ni ferrite nanomaterials. Wu et al.20 reported that Zn–Ni–Fe composite oxide nanotubes with length of 1 to 5 μm and outer diameter of 200 nm showed 60 Oe of coercivity and 0.6 emu g−1 of largest magnetocrystalline magnetization. The properties of ferrites are known to be strongly influenced by the composition, size, morphology and microstructures.21 With no doubt, Zn–Ni ferrite nanorods are expecting to show unique magnetic properties.
In this work, we report the synthesis of Zn–Ni ferrite nanorods with various magnetic properties. This was achieved by calcination of precursors with different molar ratio of Zn, Ni and Fe, prepared using a coprecipitation reaction of Zn2+, Ni2+ and Fe3+ with H2C2O4 in a microemulsion solution.
2. Experimental
2.1. Preparation of Zn–Ni ferrite nanorods
A microemulsion system consisting of cetyltrimethylammonium bromide (CTAB)/water/cyclohexane/n-pentanol was selected for synthesis of ZnxNi1−xFe2(C2O4)3 precursors. The microemulsion was prepared by dissolving CTAB (1.0 g) in a mixture of 75 ml of cyclohexane and 2.5 ml of n-pentanol. The resulting solution was stirred for 30 min. Subsequently, 3.75 ml of an oxalic acid (H2C2O4) aqueous solution (1.2 M) was added into the above solution and the mixture was stirred for an additional 1 h. 1.25 ml of an aqueous solution containing ZnSO4[0.4 × M], NiSO4[(1 − x)0.4 M] (x = 0–1) and FeSO4(0.8 M) was then added to the above microemulsion and stirred for 24 h at room temperature. The as-synthesized solid ZnxNi1−xFe2(C2O4)3 precursor was washed with alcohol and distilled water, centrifuged, and dried. Finally, ZnxNi1−xFe2O4 nanorods were obtained by calcination of the precursor at different temperatures for 3 h.
2.2. Characterization
Energy-dispersive X-ray spectroscopy (EDS) and field emission scanning electron microscopy (FESEM) were performed with a Hitachi S-4800 microscope operated at 15 kV. Thermal gravimetric analysis (TGA) was carried out on a SEIKO TG/DTA6200 thermal analyser at a heating rate of 10 K min−1 from room temperature to 800 °C in air. Powder X-ray diffraction (XRD) patterns were recorded on a X-ray diffractometer (Rigaku Ultima IV) at 40 kV and 40 mA with Cu Kα radiation. The magnetic properties were measured using a Lakeshore 7300 vibrating sample magnetometer (VSM).
3. Results and discussion
3.1. Morphology and structure of Zn–Ni–Fe composite oxalate nanorods
Fig. 1 shows SEM image and EDS spectrum of Zn0.5Ni0.5Fe2(C2O4)3 nanorods. As shown in Fig. 1a, Zn0.5Ni0.5Fe2(C2O4)3 nanorods are around 50–200 nm in diameter and several micrometers in length. The SEM image also reveals that the surface of the rods is very smooth. By observation of the EDS spectrum of the nanorods (Fig. 1b), only Zn, Ni, Fe, C and O are detected, which further confirms the formation of pure Zn0.5Ni0.5Fe2(C2O4)3 nanorods. Fig. 2 shows XRD patterns of the four different oxalate nanorods prepared at similar conditions. The diffraction peaks of Zn–Ni–Fe composite oxalate (Fig. 2a) are different from those of any other three single metal oxalate (Fig. 2b–d) and not the simple superposition of b, c and d, implying that the structure of the precursor is Zn–Ni–Fe composite oxalate like Zn0.5Ni0.5Fe2(C2O4)3.
 |
| | Fig. 1 Morphology and composition of the Zn0.5Ni0.5Fe2(C2O4)3 nanorods prepared by a microemulsion-based method: (a) SEM image, (b) EDS spectrum. | |
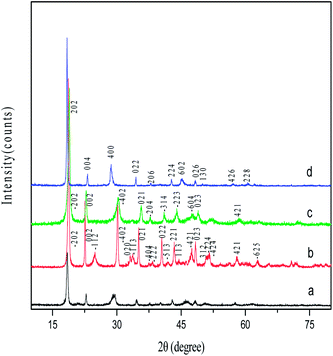 |
| | Fig. 2 XRD patterns of the different oxalate nanorods: (a) Zn0.5Ni0.5Fe2(C2O4)3, (b) ZnC2O4, (c)NiC2O4, and (d) FeC2O4. | |
3.2. Thermal property of Zn–Ni–Fe composite oxalate nanorods
As shown in Fig. 3, the thermal behavior of Zn–Ni–Fe composite oxalate nanorods is characterized by two transitions: the first one at low temperature corresponds to the loss of surface water; the second one at higher temperature (180–320 °C) is due to decomposition of the oxalate. Therefore Zn–Ni ferrite nanorods can be obtained by calcining oxalate precursors at higher than 320 °C based on this TGA curve.
 |
| | Fig. 3 TGA curve of Zn–Ni–Fe composite oxalate nanorods. | |
3.3. Morphology and structure of Zn–Ni ferrite nanorods
SEM images of Zn0.5Ni0.5Fe2O4 nanorods by calcination of the precursors at different temperatures (350, 500, 650, 800 and 900 °C) are shown in Fig. 4. Fig. 4a shows Zn0.5Ni0.5Fe2(C2O4)3 precursor nanorods which are characterized by the smooth surface morphology. After calcination, the products retain original rodlike morphology of the precursor and the Zn0.5Ni0.5Fe2O4 nanorods are formed by nanoparticles. It can be concluded that the formation of the Zn0.5Ni0.5Fe2O4 nanorods during the calcination process was restricted within the Zn0.5Ni0.5Fe2(C2O4)3 template. In other words, this growth process can be viewed as a morphologically templated nucleation process.22 As shown in Fig. 4b, the Zn0.5Ni0.5Fe2O4 nanorods obtained at 350 °C start to crystallize as the precursor Zn0.5Ni0.5Fe2(C2O4)3 begins to decompose into Zn0.5Ni0.5Fe2O4 but the nanoparticles are too little to be observed. Fig. 4c shows the SEM image of the nanorods prepared at 500 °C, small Zn0.5Ni0.5Fe2O4 nanoparticles are clearly visible. At 650 and 800 °C (Fig. 4d and e), the nanoparticles are now much larger and the number of particles is much lower, indicating an Ostwald ripening and “oriented attachment” processes and the rods are still two micrometers in length. At 900 °C, the Zn0.5Ni0.5Fe2O4 nanorods consist of individual crystals bound to each other.
 |
| | Fig. 4 (a) SEM image of Zn0.5Ni0.5Fe2(C2O4)3 nanorods; ((b)–(f)) SEM images of Zn0.5Ni0.5Fe2O4 nanorods obtained at different temperatures, (b) 350 °C, (c) 500 °C, (d) 650 °C, (e) 800 °C and (f) 900 °C. | |
Fig. 5 shows XRD patterns for the Zn0.5Ni0.5Fe2O4 nanorods prepared by decomposition of the precursor at 350, 500, 650, 800 and 900 °C respectively. It can be seen that the inverse spinel ferrite of Zn0.5Ni0.5Fe2O4 is formed as a single phase at all temperatures. All the diffraction peaks can be indexed as pure cubic phase of zinc–nickel ferrite (Zn0.5Ni0.5Fe2O4). The intensity of the diffraction peaks of Zn0.5Ni0.5Fe2O4 increases and the peak width (full width at half maximum) decreases with increasing calcinations temperatures. This indicates that, as expected and observed, both the crystallinity and the average size of the crystallites increase with increasing calcination temperatures.
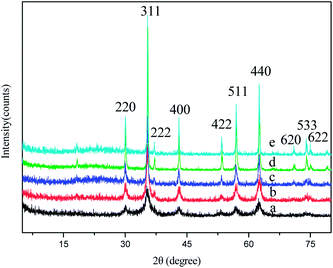 |
| | Fig. 5 XRD patterns of Zn0.5Ni0.5Fe2O4 nanorods prepared by calcination of the corresponding precursors at different temperatures (a) 350 °C, (b) 500 °C, (c) 650 °C, (d) 800 °C and (e) 900 °C. | |
Fig. 6 shows SEM images of the ZnxNi1−xFe2O4 nanorods with different x values. They are all obtained by decomposition of the precursors at 500 °C. The SEM images show rodlike morphology formed by nanoparticles and the diameter decreases with increasing Zn content. Chemical formulas for ZnxNi1−xFe2O4 nanorods are given in Table 1 based on the metal analysis data. Zn–Ni–Fe molar ratios for all samples are consistent with those used in the starting solutions, indicating the complete precipitation of metal ions.
 |
| | Fig. 6 SEM image of the ZnxNi1−xFe2O4 nanorods with different x values, (a) 0.1, (b) 0.35, (c) 0.5, (d) 0.65, and (e) 0.9. | |
Table 1 Measurement ratio of the metal elements of ZnxNi1−xFe2O4 nanorods
| Sample |
x value |
Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe Fe |
| ZnxNi1−xFe2O4 |
0.1 |
0.08![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.85 0.85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 0.35 |
0.31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.62 0.62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 0.5 |
0.54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.44 0.44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 0.65 |
0.60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.39 0.39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 0.9 |
0.86![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.07 0.07![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
3.4. Magnetic properties of Zn–Ni ferrite nanorods
Fig. 7 shows magnetic hysteresis loops of ZnxNi1−xFe2O4 (x = 0.5) nanorods prepared by calcination of the precursors at different temperatures. The saturation magnetization (Ms) are summarized in Table 2. The Ms gradually increases with increasing calcination temperatures, or more precisely the crystallite size of the particles. The sample prepared at 350 °C exhibits a narrower hysteresis loop in the M–H curve with Ms of 17.8 emu g−1. While in case of the ZnxNi1−xFe2O4 (x = 0.5) nanorods obtained at 900 °C, the Ms remarkably increases to 77.1 emu g−1. Combined with XRD and SEM results, we suggest that well crystallinity and large nanoparticle size of the sample due to the higher temperature aroused the significant increase of Ms. The lower Ms associated to the particles with smaller sizes can be attributed to two reasons. First, surface distortions due to the interaction of the transition metal ions with the oxygen atoms in the spinel lattice can reduce the net magnetic moment in the particle. This effect is especially prominent for the ultrafine particles due to their large surface to volume ratio.23 Second, the magnetocrystalline anisotropy of the particles is dependent on the crystallinity of the nanoparticles. As shown in the XRD patterns, samples prepared by decomposition of the precursor at lower temperatures exhibit the lower intensity and crystallinity. Hence, a large proportion of crystal defects and dislocations can occur within the lattice and this will cause a significant reduction of magnetic moment within the particles, as a result of the magnetocrystalline anisotropy distortion.
 |
| | Fig. 7 Magnetic hysteresis loops for ZnxNi1−xFe2O4 (x = 0.5) nanorods prepared by calcinations of the precursor at different temperatures, (a) 350 °C, (b) 500 °C, (c) 650 °C, (d) 800 °C and (e) 900 °C. | |
Table 2 Magnetic parameters for ZnxNi1−xFe2O4 (x = 0.5) nanorods prepared by calcination of the precursor at different temperatures
| Temperature (°C) |
Ms (emu g−1) |
| 350 |
17.8 |
| 500 |
51.1 |
| 650 |
56.3 |
| 800 |
63.6 |
| 900 |
77.1 |
Fig. 8 shows hysteresis loops of ZnxNi1−xFe2O4 nanorods with different x values obtained at 500 °C. As sown in Table 3, ZnxNi1−xFe2O4 nanorods with different x values show Ms of 9.3–51.1 emu g−1 due to different compositions. When Zn doping level (x value) increases from 0.1 to 0.5, the Ms of samples increases. However with further increasing x value to 0.9, the Ms of ZnxNi1−xFe2O4 nanorods decreases. The Zn0.5Ni0.5Fe2O4 nanorods show a maximum Ms of 51.1 emu g−1.
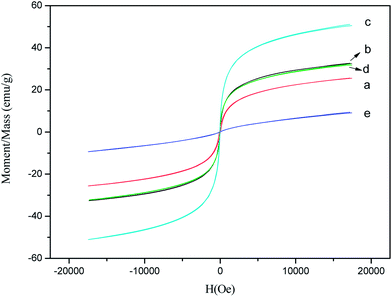 |
| | Fig. 8 Magnetic hysteresis loops for ZnxNi1−xFe2O4 nanorods with different x values, (a) 0.1, (b) 0.35, (c) 0.5, (d) 0.65, and (e) 0.9. | |
Table 3 Magnetic parameters for ZnxNi1−xFe2O4 nanorods
| Sample |
Ms (emu g−1) |
| ZnxNi1−xFe2O4 (x = 0.1) |
25.6 |
| ZnxNi1−xFe2O4 (x = 0.35) |
32.6 |
| ZnxNi1−xFe2O4 (x = 0.5) |
51.1 |
| ZnxNi1−xFe2O4 (x = 0.65) |
32.3 |
| ZnxNi1−xFe2O4 (x = 0.9) |
9.4 |
When non-magnetic divalent Zn2+ ions are introduced, they tend to occupy tetrahedral (A) sites by transferring Fe3+ ions to octahedral (B) sites due to their favoritism by polarization effect. However, site preference of cations also depends upon their electronic configurations. Zn2+ ions show marked preference for A sites where their free electrons respectively can form a covalent bond with the free electrons of the oxygen ion. This forms four bonds oriented towards the corners of a tetrahedron. Ni2+ ions have marked preference for an octahedral environment due to the favorable fit of the charge distribution of these ions in the crystal field at B sites. In view of the above considerations the cation distribution can be written as (Znx2+Fe1−x3+)A(Ni1−x2+Fe1+x3+)B.24,25 As the content of Zn2+ ions increases, the number of Fe3+ ions on B-sites increases. Therefore the magnetic moment on B-site increases and the magnetic moment on A-sites decreases. The net value of Ms increases up to x = 0.5. When ‘x’ exceeds 0.5, Ms starts decreasing because magnetic moment of the few Fe3+ ions on the A-site are no longerable to align all the moments on the B-sites antiparallel to themselves, since this is opposed by the negative B–B exchange interaction.26–29
4. Conclusions
In summary, Zn0.5Ni0.5Fe2O4 nanorods were successfully prepared by calcination of the Zn0.5Ni0.5Fe2(C2O4)3 nanorods which were synthesized by a microemulsion method. The Zn0.5Ni0.5Fe2O4 nanorods retain the original rodlike morphology of the Zn0.5Ni0.5Fe2(C2O4)3 nanorods through the whole calcination process and they have the same dimension of 50–200 nm in diameter and several micrometers in length. The magnetic properties of the Zn0.5Ni0.5Fe2O4 nanorods are largely influenced by calcination temperatures. As the calcination temperatures increase from 350 to 900 °C, the Ms of the Zn0.5Ni0.5Fe2O4 nanorods increase. The Zn0.5Ni0.5Fe2O4 nanorods obtained at 900 °C show a maximum Ms of 77.1 emu g−1. When the Zn doping level (x value) increases from 0.1 to 0.5, the Ms of samples increases. However with further increasing x value to 0.9, the Ms of ZnxNi1−xFe2O4 nanorods decreases.
Acknowledgements
This work was supported by National Natural Science Foundation of China (no. 21071017 and no. 21376029).
References
- T. J. Shinde, A. B. Gadkari and P. N. Vasambekar, J. Magn. Magn. Mater., 2013, 333, 152 CrossRef CAS.
- E. P. Muniz, J. R. C. Proveti, R. D. Pereira, B. Segatto, P. S. S. Porto, V. P. Nascimento, M. A. Schettino and E. C. Passamani, J. Mater. Sci., 2013, 48, 1543 CrossRef CAS.
- P. Sivakumar, R. Ramesh, A. Ramanand, S. Ponnusamy and C. Muthamizhchelvan, Mater. Res. Bull., 2011, 46, 2204 CrossRef CAS.
- M. G. Naseri, E. B. Saion, H. A. Ahangar, M. Hashim and A. H. Shaari, Powder Technol., 2011, 212, 80 CrossRef CAS.
- A. Ahlawat and V. G. Sathe, J. Raman Spectrosc., 2011, 42, 1087 CrossRef CAS.
- P. Sivakumar, R. Ramesh, A. Ramanand, S. Ponnusamy and C. Muthamizhchelvan, J. Mater. Sci.: Mater. Electron., 2012, 23, 1011 CrossRef CAS.
- R. H. Kodama, A. E. Berkowltz, E. J. McNiff and S. Foner, Phys. Rev. Lett., 1996, 77(2), 394 CrossRef CAS PubMed.
- J. Lopez, L. F. Gonzalez-Bahamon, J. Prado, J. C. Caicedo, G. Zambrano, M. E. Gomez, J. Esteve and P. Prieto, J. Magn. Magn. Mater., 2012, 324, 394 CrossRef CAS.
- J. Kalarus, G. Kogias, D. Holz and V. T. Zaspalis, J. Magn. Magn. Mater., 2012, 324, 2788 CrossRef CAS.
- A. P. Kazin, M. N. Rumyantseva, V. E. Prusakov, I. P. Suzdalev and A. M. Gaskov, J. Solid State Chem., 2011, 184, 2799 CrossRef CAS.
- S. Marins, T. Ogasawara and L. Tavares, J. Mater. Sci., 2011, 46, 1640 CrossRef CAS.
- K. Gheisari, S. D. Bhame, J. T. Oh and S. Javadpour, J. Supercond. Novel Magn., 2013, 26, 477 CrossRef CAS.
- C. Caizer, Mater. Sci. Eng., B, 2003, 100, 63 CrossRef.
- A. Ceylan, S. Ozcan, C. Ni and S. I. Shah, J. Magn. Magn. Mater., 2008, 320, 857 CrossRef CAS.
- S. M. Olhero, D. Soma, V. S. Amaral, T. W. Button, F. J. Alves and J. M. F. Ferreira, J. Eur. Ceram. Soc., 2012, 32, 2469 CrossRef CAS.
- M. Sertkol, Y. Koseoglu, A. Baykal, H. Kavas and A. C. Basaran, J. Magn. Magn. Mater., 2009, 321, 157 CrossRef CAS.
- Z. Yue, W. Guo, J. Zhou, Z. Gui and L. Li, J. Magn. Magn. Mater., 2004, 270, 216 CrossRef CAS.
- M. Rahimi, P. Kameli, M. Ranjbar, H. Hajihashemi and H. Salamati, J. Mater. Sci., 2013, 48, 2969 CrossRef CAS.
- K. Gheisari, S. Shahriari and S. Javadpour, J. Alloys Compd., 2013, 552, 146 CrossRef CAS.
- H. Y. Wu, Q. Z. Jiao, Y. Zhao, H. B. Liu, X. F. Li, Y. Cao and X. L. Tang, Synth. React. Inorg., Met.-Org., Nano-Met. Chem., 2010, 40, 695 CAS.
- A. Verma, T. C. Goel, R. G. Mendiratta and M. I. Alam, Mater. Sci. Eng., B, 1999, 60, 156 CrossRef.
- Z. T. Zhang, A. J. Rondinone, J. X. Ma, J. Shen and S. Dai, Adv. Mater., 2005, 17, 1415 CrossRef CAS.
- M. Rajendran, R. C. Pullar, A. K. Bhattacharya, D. Das, S. N. Chintalapudi and C. K. Majumdar, J. Magn. Magn. Mater., 2001, 232, 71 CrossRef CAS.
- A. D. Sheikh and V. L. Mathe, J. Mater. Sci., 2008, 43, 2018 CrossRef CAS.
- M. Ajmal and A. Maqsood, Mater. Lett., 2008, 62, 2077 CrossRef.
- E. W. Gorter, Nature, 1950, 165, 798 CrossRef CAS PubMed.
- C. W. Yan, Q. S. Zeng, G. F. Goya, T. Torres, J. F. Liu, H. P. Wu, M. Y. Ge, Y. W. Zeng, Y. W. Wang and J. Z. Jiang, J. Phys. Chem. C, 2007, 111, 12274 Search PubMed.
- M. A. Ahmed and M. M. El-Sayed, J. Magn. Magn. Mater., 2007, 308, 40 CrossRef CAS.
- J. T. Wu, N. Li, J. Xu, Y. Q. Jiang, Z. G. Ye, Z. X. Xie and L. S. Zheng, Appl. Phys. Lett., 2011, 99, 202505 CrossRef.
|
| This journal is © The Royal Society of Chemistry 2014 |
Click here to see how this site uses Cookies. View our privacy policy here. 



![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni
Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe
Fe![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.85
0.85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.62
0.62![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.44
0.44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.39
0.39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.07
0.07![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2



