Total syntheses of rubiginone A2, C2, and fujianmycin A†
Devendar G. Vanga and
Krishna P. Kaliappan*
Department of Chemistry, Indian Institute of Technology Bombay, Powai, Mumbai-400 076, India. E-mail: kpk@chem.iitb.ac.in; Fax: +91 22-2572-3480; Tel: +91 22-2576-7177
First published on 14th January 2014
Abstract
The total syntheses of rubiginone A2, C2 and fujianmycin A are described. The synthesis involves Diels–Alder/aromatization and photo chemical reactions as key steps to construct the tetracyclic frame of benz[a]anthraquinone skeleton. Sharpless epoxidation, a copper-catalyzed regioselective epoxide opening, and enyne metathesis reactions are utilized as the key steps for the synthesis of chiral vinylcyclohexene.
Introduction
Angucycline/angucyclinone antibiotics,1–3 ever-growing natural products having benz[a]anthraquinone skeleton, are biogenetically categorized as aromatic polyketides catalyzed by the polyketide synthases (PKS), namely type II PKS. They have been shown to display a broad spectrum of biological properties such as antitumor, antifungal, antiviral properties, platelet aggregation and enzyme inhibitory behavior.4 Although the isolation of these natural products started in 1966, a tremendous improvement in the development of angucyclinone antibiotic natural products has taken place only in the last two decades. As a result of their remarkable biological profile and low availability from microorganisms, many synthetic chemists have been actively involved in the synthesis of these natural products. Several synthetic strategies have been developed to construct the benz[a]anthraquinone skeleton of angucyclinones employing Diels–Alder reaction, nucleophilic addition, electrophilic addition (free radical annulations), transition metal catalyzed cross coupling and intramolecular cyclization as a key reaction. Among all these strategies, the Diels–Alder approach has been widely used to assemble the benz[a]anthraquinone skeleton5–10 by using either chiral catalyst, enantiopure diene or chiral dienophile. One of the important advantages of the Diels–Alder reaction is the ease of predicting the regioselective outcome of the product by changing substituents at the quinone moiety. Apart from its operational simplicity, the other benefit of the Diels–Alder reaction is its convergent approach, which allows introducing diversity in the molecules while planning the synthesis of simpler analogues of angucyclinones. Particularly, rubiginones and fujianmycin A are interesting angucyclinone antibiotics due to the presence of a C-4 hydroxyl group which makes them special from the rest of their family as evident from the literature that only one total synthesis has been reported for these molecules.11 Rubiginone Al (1), C1 (2), A2 (3) and C2 (4), which differ from one another in the oxidation pattern at C-1 and C-4, and acylation at the C-4 hydroxy group, were isolated from the fermentation broth of Streptomyces griseorubiginosus. They exhibit potentiation of vincristine-induced cytotoxicity against multi-drug-resistant tumor cells.12 Furthermore, rubiginone A2 (3), also known as fujianmycin B13 or SNA-8093-B,14 has been found to be useful in the treatment of AIDS and Alzheimer's disease.15 In 1990, Konishi et al. reported the absolute stereochemistry of rubiginones by NMR spectral analysis using the O-methylmandelate method.16 Fujianmycin A (5), that varies from rubiginone A2 (3) at C-8 functionality, was isolated from the inoculum of the Streptomyces sp. (IA-CAS-114) in 1985 by Richards et al.13 It was found to display antibacterial activity against Bacillus subtilis at 50 μg mL−1. On the other hand, its methyl derivative rubiginone A2 (3) was active only at much higher concentration indicating that the presence of C-8 phenolic functional is important for the microbial activity (Fig. 1).In connection with our interest in the total syntheses of angucyclinone antibiotics,17 we became interested in the synthesis of rubiginone A2, C2 and fujianmycin A. Herein we report a unified strategy for the enantioselective syntheses of these molecules by employing a strategy that involves a sequential enyne metathesis, Diels–Alder reaction and a photo chemical reaction as key steps.
Results and discussion
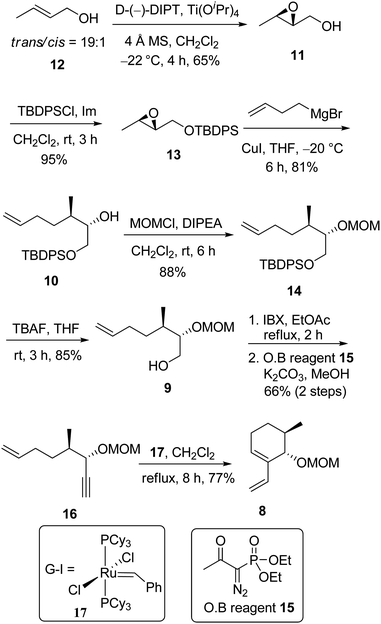
Most of the synthetic approaches, including ours, to angucyclinones have relied mainly on Diels–Alder reaction and not surprisingly, our retrosynthetic plan for the synthesis of rubiginones and fujianmycin A has also been based on Diels–Alder reaction. As shown in Scheme 1, we envisioned that the synthesis of rubiginone A1 and C1, in principle, could be achieved via a reduction of C-1 keto group of rubiginone A2 and C2. It was anticipated that the use of the Diels–Alder reaction, aromatization protocol and photooxygenation would facilitate the total synthesis of rubiginone A2 3, C2 4, and fujianmycin A 5 from naphthoquinones 7 and enantioenriched vinylcyclohexene 8. It was also envisaged that the vinylcyclohexene 8 could be generated from the oxidation of primary alcohol 9 followed by treatment with Ohira–Bestmann reagent and intramolecular enyne metathesis reaction.The primary alcohol 9 could then be accessed from compound 10 by protecting group manipulation. Working further back, the compound 10 could be obtained by a copper catalyzed regioselective opening of epoxide 11.
With this retrosynthetic plan in mind, our initial focus was to synthesize the common enantioenriched vinylcyclohexene 8 which has the required stereocenters in all target molecules. As shown in Scheme 2, the synthesis commenced with Sharpless epoxidation18 of crotyl alcohol 12 (trans/cis = 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to give the known epoxide 11 (ref. 19) in 65% yield. The epoxy alcohol was protected as its silyl ether with TBDPSCl to afford compound 13 in 95% yield. A copper-catalyzed20 addition of homo allyl magnesium bromide to epoxide 13 afforded 9
1) to give the known epoxide 11 (ref. 19) in 65% yield. The epoxy alcohol was protected as its silyl ether with TBDPSCl to afford compound 13 in 95% yield. A copper-catalyzed20 addition of homo allyl magnesium bromide to epoxide 13 afforded 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of separable regioisomeric alcohols in favor of the required isomer 10 in 81% yield. The major alcohol 10 was protected as its MOM ether 14 in 88% yield and subsequent removal of TBDPS group furnished the primary alcohol 9 in 85% yield. For the homologation, the primary alcohol 9 was oxidized with IBX, followed by treatment with Bestmann–Ohira reagent 15 to provide the enyne 16. The stage was now set to synthesize enantioenriched diene 8 from enyne 16. As anticipated, when the enyne 16 was subjected to intramolecular enyne metathesis with Grubbs' first generation catalyst (G-I) 17 (ref. 21) (7 mmol%), the desired enantioenriched vinylcyclohexene 8 was obtained in 77% yield.
1 ratio of separable regioisomeric alcohols in favor of the required isomer 10 in 81% yield. The major alcohol 10 was protected as its MOM ether 14 in 88% yield and subsequent removal of TBDPS group furnished the primary alcohol 9 in 85% yield. For the homologation, the primary alcohol 9 was oxidized with IBX, followed by treatment with Bestmann–Ohira reagent 15 to provide the enyne 16. The stage was now set to synthesize enantioenriched diene 8 from enyne 16. As anticipated, when the enyne 16 was subjected to intramolecular enyne metathesis with Grubbs' first generation catalyst (G-I) 17 (ref. 21) (7 mmol%), the desired enantioenriched vinylcyclohexene 8 was obtained in 77% yield.
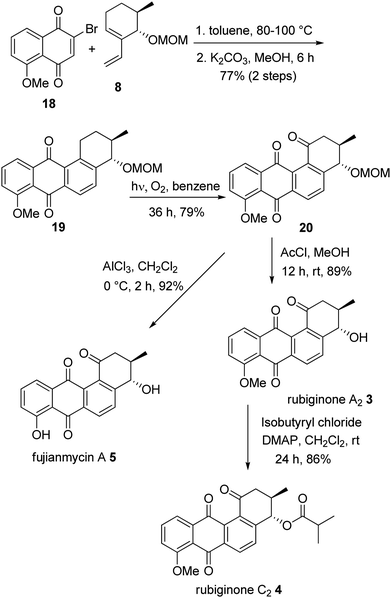
With the key intermediate vinylcyclohexene 8 in hand, we then focused on the syntheses of rubiginone A2, C2 and fujianmycin A. In order to accomplish the syntheses of 3, 4 and 5, we selected 5-methoxy-2-bromonaphthoquinone 18 as a dienophile. Accordingly, the vinylcyclohexene 8 was treated with 18 at 80 °C for 12 h, followed by aromatization with K2CO3 and MeOH to afford the tetracyclic compound 19 in 77% yield for two steps. A highly regioselective photochemical oxidation17 provided the ketone 20 in good yield which was then treated with AlCl3 to smoothly cleave both MOM and methyl ethers to furnish fujianmycin A 5 in excellent yield. On the other hand, selective cleavage of MOM group was successfully achieved by treating with AcCl/MeOH to afford rubiginone A2 3 in 89% yield. The other natural product rubiginone C2 4 was easily synthesized by treating 3 with isobutyryl chloride and DMAP in CH2Cl2 in 86% yield. The spectral data of 3, 4 and 5 were found to be identical in all aspects with that of natural products and we have accomplished the total syntheses of rubiginone A2 3 in 14% overall yield (10 steps), rubiginone C2 4 in 12% overall yield (11 steps) and fujianmycin A 5 in 16% overall yield (10 steps) (Scheme 3).
Conclusions
In summary, we have successfully accomplished the enantioselective total synthesis of the C-4 oxygenated angucyclinones rubiginones A2 3, C2 4, and fujianmycin A 5 by employing Diels–Alder reaction, aromatization and photooxygenation as key steps. The key intermediate, chiral vinylcyclohexene 8, was successfully synthesized in 22% overall yield from known compound 11 by employing Sharpless epoxidation, a copper-catalyzed regioselective epoxide opening and enyne metathesis reactions as key steps. The regioselective construction of the tetracyclic skeleton of natural angucyclinones (+)-3, (−)-4 and (+)-5 was achieved via a stereocontrolled Diels–Alder reaction between 8 and 5-methoxy-2-bromo naphthoquinone 18. Aromatization and photooxygenation allowed the enantioselective total syntheses of rubiginone A2, C2 and fujianmycin A in 14, 12 and 16% overall yield, respectively, from 11.Experimental section
General methods
Unless otherwise noted, all starting materials and reagents were obtained from commercial suppliers and used after further purification. Tetrahydrofuran was distilled from sodium benzophenone ketyl and toluene from sodium. Dichloromethane, N,N-dimethylformamide, hexanes and pyridine were freshly distilled from calcium hydride. All solvents for routine isolation of products and chromatography were of reagent grade and glass distilled. Reaction flasks were dried in oven at 100 °C for 12 h. Air and moisture sensitive reactions were performed under an argon/UHP nitrogen atmosphere. Chromatography was performed using silica gel (100–200 mesh, Aceme, for gravity column chromatograph; 230–400 mesh, Aceme, for Biotage flash column chromatography) with indicated solvents. All reactions were monitored by thin-layer chromatography carried out on 0.25 mm E. Merck silica plates (60F-254) using UV light as visualizing agent, and charring solution (prepared by drop wise addition of Conc. H2SO4 (5 mL) to a solution of phosphomolybdic acid (1 g) and ceric sulphate (2 g) in water (95 mL)), alkaline KMnO4 solution (prepared by dissolving KMnO4 (2 g) and NaHCO3 (4 g) in water (100 mL)), and heat as developing agents. Optical rotation was recorded on Autopol IV automatic polarimeter. IR spectra were recorded on Thermo Nicolet Avater 320 FT-IR and Nicolete Impact 400 machine. Mass spectra were obtained from Waters Micromass-Q-Tof micro™ (YA105) spectrometer. 1H and 13C NMR spectra were recorded either on Varian AS 400, Varian ASM 300 or Bruker 400. NMR data is in the order of chemical shifts, multiplicity (s, singlet; br s, broad singlet; d, doublet; t, triplet; q, quartet; m, multiplet), number of protons and coupling constant in hertz (Hz).((2R,3R)-3-methyloxiran-2-yl)methanol (11)18
A suspension of 4 Å MS (4 g) in 200 mL of CH2Cl2 at room temperature was treated with D-(−)-diisopropyl tartrate (1.42 g, 6.0 mmol) and titanium tetraisopropoxide (1.42 g, 5.0 mmol) and the mixture was cooled to −20 °C. A solution of alcohol 12 (trans/cis = 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (7.21 g, 100.0 mmol) in CH2Cl2 (25 mL) was added to this mixture, which was stirred at the same temperature for 40 min. Dry tert-butyl hydroperoxide (39.4 mL, 3.8 M solution in toluene, 150 mmol) was added dropwise for 20 min through a syringe pump and the stirring was continued for an additional 3 h. The reaction mixture was then warmed to 0 °C and treated with water (15 mL). Finally, the mixture was warmed to room temperature and treated with a 30% solution of NaOH in saturated NaCl solution (3.5 mL). The mixture was stirred at room temperature for 1 h. Then the reaction mixture was poured into a solution of 10% acetone/diethyl ether in 1 g citric acid in 150 mL and stirred for 1 h. After filtration through a pad of Celite the filtrate was concentrated, and purified by silica gel column chromatography (40% ethyl acetate in hexanes) to afford the epoxy alcohol 11 (5.7 g, 65%) as a colorless oil; Rf = 0.5 (1
1) (7.21 g, 100.0 mmol) in CH2Cl2 (25 mL) was added to this mixture, which was stirred at the same temperature for 40 min. Dry tert-butyl hydroperoxide (39.4 mL, 3.8 M solution in toluene, 150 mmol) was added dropwise for 20 min through a syringe pump and the stirring was continued for an additional 3 h. The reaction mixture was then warmed to 0 °C and treated with water (15 mL). Finally, the mixture was warmed to room temperature and treated with a 30% solution of NaOH in saturated NaCl solution (3.5 mL). The mixture was stirred at room temperature for 1 h. Then the reaction mixture was poured into a solution of 10% acetone/diethyl ether in 1 g citric acid in 150 mL and stirred for 1 h. After filtration through a pad of Celite the filtrate was concentrated, and purified by silica gel column chromatography (40% ethyl acetate in hexanes) to afford the epoxy alcohol 11 (5.7 g, 65%) as a colorless oil; Rf = 0.5 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ethyl acetate/hexanes); [α]22D = +45.04 (c 1.0, CHCl3); IR (CHCl3): 3401, 2978, 2929, 2875, 1453, 1385, 1218, 1102, 1041, 865 cm−1; 1H NMR (CDCl3, 400 MHz): δ 3.90–3.83 (m, 1H), 3.65–3.58 (m, 1H), 3.04–3.01 (m, 1H), 2.91–2.88 (m, 1H), 1.32 (d, J = 5.6 Hz, 3H); 13C NMR (CDCl3, 100 MHz): δ 61.8, 59.7, 52.2, 17.3; HRMS (ESI): calcd. for C4H8O2Na (M + Na)+ m/z 111.0422, found m/z 111.0424.
1 ethyl acetate/hexanes); [α]22D = +45.04 (c 1.0, CHCl3); IR (CHCl3): 3401, 2978, 2929, 2875, 1453, 1385, 1218, 1102, 1041, 865 cm−1; 1H NMR (CDCl3, 400 MHz): δ 3.90–3.83 (m, 1H), 3.65–3.58 (m, 1H), 3.04–3.01 (m, 1H), 2.91–2.88 (m, 1H), 1.32 (d, J = 5.6 Hz, 3H); 13C NMR (CDCl3, 100 MHz): δ 61.8, 59.7, 52.2, 17.3; HRMS (ESI): calcd. for C4H8O2Na (M + Na)+ m/z 111.0422, found m/z 111.0424.
Tert-butyl (((2R,3R)-3-methyloxiran-2-yl)methoxy)diphenylsilane (13)
To a solution of epoxy alcohol 11 (2.50 g, 28.4 mmol) in dry CH2Cl2 was added imidazole (3.86 g, 56.8 mmol) at 0 °C under N2 atmosphere. To this mixture was added TBDPSCl (8.6 g, 31.2 mmol) drop wise and then the reaction mixture was slowly brought to room temperature and stirred for further 3 h. The reaction mixture was quenched with water, and extracted with CH2Cl2 (2 × 50 mL). The organic layer was washed with brine, dried over Na2SO4, concentrated, and purified by silica gel column chromatography (3–5% ethyl acetate in hexanes) to afford the silyl ether 13 (8.8 g, 95%) as a colorless liquid; Rf = 0.7 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 ethyl acetate/hexanes); [α]22D = +6.73 (c 0.6, CHCl3); IR (CHCl3): 3065, 2956, 2931, 2858, 1590, 1459, 1428, 1113, 1026, 741 cm−1; 1H NMR (CDCl3, 400 MHz): δ 7.70–7.76 (m, 4H), 7.45–7.36 (m, 6H), 3.80–3.71 (m, 2H), 2.88–2.84 (m, 2H), 1.29 (d, J = 4.8 Hz, 3H), 1.05 (s, 9H); 13C NMR (CDCl3, 100 MHz): δ 135.8, 135.7, 133.5, 129.9, 127.9, 64.2, 59.6, 52.4, 26.9, 19.4, 17.5; HRMS (ESI): calcd. for C20H26O2SiNa (M + Na)+ m/z 349.1600, found m/z 349.1601.
9 ethyl acetate/hexanes); [α]22D = +6.73 (c 0.6, CHCl3); IR (CHCl3): 3065, 2956, 2931, 2858, 1590, 1459, 1428, 1113, 1026, 741 cm−1; 1H NMR (CDCl3, 400 MHz): δ 7.70–7.76 (m, 4H), 7.45–7.36 (m, 6H), 3.80–3.71 (m, 2H), 2.88–2.84 (m, 2H), 1.29 (d, J = 4.8 Hz, 3H), 1.05 (s, 9H); 13C NMR (CDCl3, 100 MHz): δ 135.8, 135.7, 133.5, 129.9, 127.9, 64.2, 59.6, 52.4, 26.9, 19.4, 17.5; HRMS (ESI): calcd. for C20H26O2SiNa (M + Na)+ m/z 349.1600, found m/z 349.1601.
(2S,3R)-1-(tert-butyldiphenylsilyloxy)-3-methylhept-6-en-2-ol (10)
A solution of homoallyl magnesium bromide (prepared from 3.21 mL of homoallyl bromide, 61.2 mmol) in THF (30 mL) was added to a solution of CuI (407 mg, 2.14 mmol) in THF (20 mL) at −25 °C. After stirring for 15 min. a solution of silyl ether 13 (7.0 g, 21.4 mmol) was added dropwise to the reaction mixture and stirred for 6 h at same temperature. The reaction mixture was quenched with aq. NH4Cl solution and then the aqueous layer was extracted with ether (3 × 30 mL). The combined organic layers were washed with brine, dried over Na2SO4, and solvent was evaporated under vacuo. The crude compound was subjected to purification by silica gel column chromatography (10–15% ethyl acetate in hexanes) to afford alcohol 10 (2.4 g, 81%) as a colourless liquid; Rf = 0.68 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 ethyl acetate/hexanes); [α]22D = +3.95 (c 2.5, CHCl3); IR (CHCl3): 3461, 2961, 2931, 2859, 1640, 1590, 1428, 1217, 1113, 1039, 760 cm−1; 1H NMR (CDCl3, 400 MHz): δ 7.68–7.64 (m, 4H), 7.46–7.36 (m, 6H), 5.85–5.74 (m, 1H), 4.97 (d, J = 17.6 Hz, 1H), 4.91 (d, J = 10.2 Hz, 1H), 3.69–3.58 (m, 3H), 2.12–1.94 (m, 2H), 1.59–1.52 (m, 1H), 1.51–1.43 (m, 1H), 1.22–1.14 (m, 1H), 1.06 (s, 9H), 0.89 (d, J = 6.8 Hz, 3H); 13C NMR (CDCl3, 100 MHz): δ 138.9, 135.8, 135.7, 133.3, 130.0, 129.9, 129.8, 128.0, 127.9, 114.6, 75.0, 66.4, 34.7, 32.3, 31.4, 27.0, 26.9, 19.3, 14.5; HRMS (ESI): calcd. for C24H34O2SiNa (M + Na)+ m/z 405.2226, found m/z 405.2225.
9 ethyl acetate/hexanes); [α]22D = +3.95 (c 2.5, CHCl3); IR (CHCl3): 3461, 2961, 2931, 2859, 1640, 1590, 1428, 1217, 1113, 1039, 760 cm−1; 1H NMR (CDCl3, 400 MHz): δ 7.68–7.64 (m, 4H), 7.46–7.36 (m, 6H), 5.85–5.74 (m, 1H), 4.97 (d, J = 17.6 Hz, 1H), 4.91 (d, J = 10.2 Hz, 1H), 3.69–3.58 (m, 3H), 2.12–1.94 (m, 2H), 1.59–1.52 (m, 1H), 1.51–1.43 (m, 1H), 1.22–1.14 (m, 1H), 1.06 (s, 9H), 0.89 (d, J = 6.8 Hz, 3H); 13C NMR (CDCl3, 100 MHz): δ 138.9, 135.8, 135.7, 133.3, 130.0, 129.9, 129.8, 128.0, 127.9, 114.6, 75.0, 66.4, 34.7, 32.3, 31.4, 27.0, 26.9, 19.3, 14.5; HRMS (ESI): calcd. for C24H34O2SiNa (M + Na)+ m/z 405.2226, found m/z 405.2225.
(S)-5-((R)-hex-5-en-2-yl)-9,9-dimethyl-8,8-diphenyl-2,4,7-trioxa-8-siladecane (14)
To a stirred solution of alcohol 10 (1 g, 2.6 mmol) and DIPEA (3.6 mL, 20.9 mmol) in CH2Cl2 (30 mL) at 0 °C was added dropwise MOMCl (4.5 mL, 3 M in toluene, 15.6 mmol) and the reaction mixture was stirred for 6 h at room temperature. The reaction mixture was quenched with water, and extracted with CH2Cl2 (3 × 30 mL). The organic layer was washed with brine, dried over Na2SO4, and concentrated under vacuo. The crude compound was purified by silica gel column chromatography (5–8% ethyl acetate in hexanes) to afford 14 (981 mg, 88%) as a colourless liquid; Rf = 0.72 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 ethyl acetate/hexanes); [α]22D = +2.71 (c 3.8, CHCl3); IR (CHCl3): 3071, 3016, 2931, 2859, 1662, 1428, 1216, 1113, 1037, 917, 760 cm−1; 1H NMR (CDCl3, 400 MHz): δ 7.68–7.65 (m, 4H), 7.44–7.36 (m, 6H), 5.83–5.73 (m, 1H), 4.99 (d, J = 17.0 Hz, 1H), 4.93 (d, J = 10.3 Hz, 1H), 4.78 (d, J = 6.8 Hz, 1H), 4.64 (d, J = 6.8 Hz, 1H), 3.74–3.70 (m, 1H), 3.64–3.58 (m, 2H), 3.34 (s, 3H), 2.11–2.02 (m, 2H), 1.59–1.53 (m, 1H), 1.29–1.20 (m, 2H), 1.04 (s, 9H), 0.83 (d, J = 6.8 Hz, 3H); 13C NMR (CDCl3, 100 MHz): δ 139.1, 135.8, 135.7, 133.6, 129.8, 129.8, 127.8, 114.5, 96.9, 81.4, 64.7, 55.8, 33.9, 32.5, 31.7, 27.0, 19.3, 14.4; HRMS (ESI): calcd. for C26H38O3SiK (M + K)+ m/z 465.2227, found m/z 465.2232.
9 ethyl acetate/hexanes); [α]22D = +2.71 (c 3.8, CHCl3); IR (CHCl3): 3071, 3016, 2931, 2859, 1662, 1428, 1216, 1113, 1037, 917, 760 cm−1; 1H NMR (CDCl3, 400 MHz): δ 7.68–7.65 (m, 4H), 7.44–7.36 (m, 6H), 5.83–5.73 (m, 1H), 4.99 (d, J = 17.0 Hz, 1H), 4.93 (d, J = 10.3 Hz, 1H), 4.78 (d, J = 6.8 Hz, 1H), 4.64 (d, J = 6.8 Hz, 1H), 3.74–3.70 (m, 1H), 3.64–3.58 (m, 2H), 3.34 (s, 3H), 2.11–2.02 (m, 2H), 1.59–1.53 (m, 1H), 1.29–1.20 (m, 2H), 1.04 (s, 9H), 0.83 (d, J = 6.8 Hz, 3H); 13C NMR (CDCl3, 100 MHz): δ 139.1, 135.8, 135.7, 133.6, 129.8, 129.8, 127.8, 114.5, 96.9, 81.4, 64.7, 55.8, 33.9, 32.5, 31.7, 27.0, 19.3, 14.4; HRMS (ESI): calcd. for C26H38O3SiK (M + K)+ m/z 465.2227, found m/z 465.2232.
(2S,3R)-2-(methoxymethoxy)-3-methylhept-6-en-1-ol (9)
To a stirred solution of silyl ether 14 (8 g, 18.7 mmol) in THF (50 mL) at 0 °C was added dropwise a THF solution of TBAF (20.6 mL, 1 M in THF, 20.6 mmol) and the reaction mixture was stirred for 3 h at room temperature. The reaction mixture was quenched with water and extracted with EtOAc (3 × 30 mL). The organic layer was washed with brine, dried over Na2SO4, and concentrated under vacuo. The crude compound was purified by silica gel column chromatography (20–30% ethyl acetate in hexanes) to afford 9 (3 g, 85%) as a colourless liquid; Rf = 0.35 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 ethyl acetate/hexanes); [α]22D = +52.50 (c 1.9, CHCl3); IR (CHCl3): 3431, 3077, 2931, 2890, 1641, 1462, 1382, 1214, 1152, 1104, 1036, 913 cm−1; 1H NMR (CDCl3, 400 MHz): δ 5.84–5.74 (m, 1H), 5.01 (d, J = 17.0 Hz, 1H), 4.95 (d, J = 10.2 Hz, 1H), 4.75 (d, J = 6.8 Hz, 1H), 4.65 (d, J = 6.8 Hz, 1H), 3.60–3.44 (m, 2H), 3.43 (s, 3H), 3.42–3.39 (m, 1H), 3.30–3.25 (m, 1H), 2.17–2.09 (m, 1H), 2.08–1.96 (m, 1H), 1.73–1.66 (m, 1H), 1.60–1.51 (m, 1H), 1.28–1.19 (m, 1H), 0.91 (d, J = 6.8 Hz, 3H); 13C NMR (CDCl3, 100 MHz): δ 138.8, 114.7, 97.9, 86.8, 64.3, 55.8, 35.1, 32.3, 31.6, 14.9; HRMS (ESI): calcd. for C10H20O3Na (M + Na)+ m/z 211.1310, found m/z 211.1317.
9 ethyl acetate/hexanes); [α]22D = +52.50 (c 1.9, CHCl3); IR (CHCl3): 3431, 3077, 2931, 2890, 1641, 1462, 1382, 1214, 1152, 1104, 1036, 913 cm−1; 1H NMR (CDCl3, 400 MHz): δ 5.84–5.74 (m, 1H), 5.01 (d, J = 17.0 Hz, 1H), 4.95 (d, J = 10.2 Hz, 1H), 4.75 (d, J = 6.8 Hz, 1H), 4.65 (d, J = 6.8 Hz, 1H), 3.60–3.44 (m, 2H), 3.43 (s, 3H), 3.42–3.39 (m, 1H), 3.30–3.25 (m, 1H), 2.17–2.09 (m, 1H), 2.08–1.96 (m, 1H), 1.73–1.66 (m, 1H), 1.60–1.51 (m, 1H), 1.28–1.19 (m, 1H), 0.91 (d, J = 6.8 Hz, 3H); 13C NMR (CDCl3, 100 MHz): δ 138.8, 114.7, 97.9, 86.8, 64.3, 55.8, 35.1, 32.3, 31.6, 14.9; HRMS (ESI): calcd. for C10H20O3Na (M + Na)+ m/z 211.1310, found m/z 211.1317.
(5R,6S)-6-(methoxymethoxy)-5-methyloct-1-en-7-yne (16)
A solution of alcohol 9 (1 g, 5.32 mmol) in ethyl acetate (20 mL) was treated with IBX (2.95 g, 10.64 mmol) at once and the reaction mixture was refluxed for 2 h. The reaction mixture was filtered off, then the filtrate was evaporated under vacuo to give crude aldehyde which was dissolved in dry MeOH and added dropwise without any further purification to a solution of Bestmann–Ohira reagent 15 (1.28 g, 5.85 mmol) and K2CO3 (1 g, 7.96 mmol) in MeOH (20 mL) under 0 °C. The reaction mixture was allowed to stir at room temperature for 4 h and quenched with water. The reaction mixture was extracted with ethyl acetate. The organic layer was washed with brine, dried over Na2SO4, concentrated, and purified by silica gel column chromatography (5–10% ethyl acetate in hexanes) to afford the enyne 16 (638 mg, 66%) as a colorless oil; Rf = 0.7 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 ethyl acetate/hexanes); [α]22D = −76.80 (c 0.3, CHCl3); IR (CHCl3): 3065, 2925, 2854, 2109, 1661, 1599, 1463, 1217, 1155, 1098, 1036, 760 cm−1; 1H NMR (CDCl3, 400 MHz): δ 5.86–5.76 (m, 1H), 5.03 (d, J = 17.0 Hz, 1H), 4.96 (d, J = 10.2 Hz, 1H), 4.95 (d, J = 6.8 Hz, 1H), 4.59 (d, J = 6.8 Hz, 1H), 4.23 (dd, J = 4.8, 2.0 Hz, 1H), 3.38 (s, 3H), 2.40 (d, J = 2.0 Hz, 1H), 2.19–2.14 (m, 1H), 2.08–2.01 (m, 1H), 1.85–1.78 (m, 1H), 1.77–1.68 (m, 1H), 1.40–1.31(m, 1H), 1.03 (d, J = 6.8 Hz, 3H); 13C NMR (CDCl3, 100 MHz): δ 138.9, 114.7, 94.4, 94.3, 81.7, 74.3, 69.8, 55.9, 37.5, 31.4, 15.3; HRMS (ESI): calcd. for C11H18O2K (M + K)+ m/z 221.0944, found m/z 221.0936.
9 ethyl acetate/hexanes); [α]22D = −76.80 (c 0.3, CHCl3); IR (CHCl3): 3065, 2925, 2854, 2109, 1661, 1599, 1463, 1217, 1155, 1098, 1036, 760 cm−1; 1H NMR (CDCl3, 400 MHz): δ 5.86–5.76 (m, 1H), 5.03 (d, J = 17.0 Hz, 1H), 4.96 (d, J = 10.2 Hz, 1H), 4.95 (d, J = 6.8 Hz, 1H), 4.59 (d, J = 6.8 Hz, 1H), 4.23 (dd, J = 4.8, 2.0 Hz, 1H), 3.38 (s, 3H), 2.40 (d, J = 2.0 Hz, 1H), 2.19–2.14 (m, 1H), 2.08–2.01 (m, 1H), 1.85–1.78 (m, 1H), 1.77–1.68 (m, 1H), 1.40–1.31(m, 1H), 1.03 (d, J = 6.8 Hz, 3H); 13C NMR (CDCl3, 100 MHz): δ 138.9, 114.7, 94.4, 94.3, 81.7, 74.3, 69.8, 55.9, 37.5, 31.4, 15.3; HRMS (ESI): calcd. for C11H18O2K (M + K)+ m/z 221.0944, found m/z 221.0936.
(5R,6S)-6-(methoxymethoxy)-5-methyl-1-vinylcyclohex-1-ene (8)
A solution of enyne 16 (300 mg, 1.65 mmol) in CH2Cl2 (40 mL) was treated with Grubb's first generation catalyst 17 (100 mg, 7 mol%) and then refluxed for 8 h. The reaction mixture was cooled to room temperature and stirred with DMSO (50 equiv. with respect to the catalyst) for 6 h to remove the metal impurities. Evaporation of the solvent and purification by silica gel column chromatography (10% ethyl acetate in hexanes) yielded 1,3-diene 8 (231 mg, 77%) as a colorless liquid; Rf = 0.68 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 ethyl acetate/hexanes); [α]22D = −11.26 (c 2.0, CHCl3); IR (CHCl3): 3005, 2929, 2851, 1646, 1450, 1214, 1150, 1096, 1040, 924, 757 cm−1; 1H NMR (CDCl3, 400 MHz): δ 6.31 (dd, J = 17.6, 11.0 Hz, 1H), 5.92 (t, J = 3.9 Hz, 1H), 5.34 (d, J = 17.6 Hz, 1H), 4.98 (d, J = 11.0 Hz, 1H), 4.82 (d, J = 7.1 Hz, 1H), 4.67 (d, J = 7.1 Hz, 1H), 4.04 (d, J = 2.0 Hz, 1H), 3.40 (s, 3H), 2.22–2.10 (m, 3H), 2.01–1.92 (m, 1H), 1.44–1.38 (m, 1H), 0.88 (d, J = 7.2 Hz, 3H); 13C NMR (CDCl3, 100 MHz): δ 138.7, 133.8, 132.6, 111.1, 95.8, 74.1, 56.1, 30.4, 22.5, 21.8, 15.3; HRMS (ESI): calcd. for C11H18O2K (M + K)+ m/z 221.0944, found m/z 221.0945.
9 ethyl acetate/hexanes); [α]22D = −11.26 (c 2.0, CHCl3); IR (CHCl3): 3005, 2929, 2851, 1646, 1450, 1214, 1150, 1096, 1040, 924, 757 cm−1; 1H NMR (CDCl3, 400 MHz): δ 6.31 (dd, J = 17.6, 11.0 Hz, 1H), 5.92 (t, J = 3.9 Hz, 1H), 5.34 (d, J = 17.6 Hz, 1H), 4.98 (d, J = 11.0 Hz, 1H), 4.82 (d, J = 7.1 Hz, 1H), 4.67 (d, J = 7.1 Hz, 1H), 4.04 (d, J = 2.0 Hz, 1H), 3.40 (s, 3H), 2.22–2.10 (m, 3H), 2.01–1.92 (m, 1H), 1.44–1.38 (m, 1H), 0.88 (d, J = 7.2 Hz, 3H); 13C NMR (CDCl3, 100 MHz): δ 138.7, 133.8, 132.6, 111.1, 95.8, 74.1, 56.1, 30.4, 22.5, 21.8, 15.3; HRMS (ESI): calcd. for C11H18O2K (M + K)+ m/z 221.0944, found m/z 221.0945.
(3R,4S)-8-methoxy-4-(methoxymethoxy)-3-methyl-1,2,3,4-tetrahydrotetraphene-7,12-dione (19)
A solution of 5-methoxy-2-bromo-1,4-naphthoquinone 18 (315 mg, 1.21 mmol) and diene 8 (200 mg, 1.1 mmol) in toluene was heated at 80 °C for 12 h and then at 100 °C for 2 h. After completion of the reaction, solvent was removed in vacuo, the crude Diels–Alder adduct was dissolved in MeOH (10 mL), treated with solid K2CO3 (760 mg, 5.5 mmol), and stirred in dark for 6 h. The reaction mixture was quenched with water, extracted with ethyl acetate, and then the combined organic layer was washed with brine, dried over Na2SO4, concentrated. The crude was purified by silica gel column chromatography (40% ethyl acetate in hexanes) to afford the tetracycle 19 (310 mg, 77%) as an orange solid; Rf = 0.5 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ethyl acetate/hexanes); mp = 208–211 °C; [α]22D = −34.0 (c 1.0, CHCl3); IR (CHCl3): 3020, 2928, 2884, 1669, 1587, 1468, 1448, 1269, 1217, 1087, 1030, 769 cm−1; 1H NMR (CDCl3, 400 MHz): δ 8.19 (d, J = 8.0 Hz, 1H), 7.85 (d, J = 6.8 Hz, 1H), 7.76 (d, J = 8.0 Hz, 1H), 7.69 (t, J = 8.0 Hz, 1H), 7.28 (d, J = 8.5 Hz, 1H), 4.85 (d, J = 6.8 Hz, 1H), 4.74 (d, J = 6.8 Hz, 1H), 4.40 (d, J = 4.8 Hz, 1H), 4.04 (s, 3H), 3.47 (s, 3H), 3.48–3.40 (m, 1H), 3.34–3.25 (m, 1H), 2.24–2.10 (m, 2H), 1.70–1.62 (m, 1H), 1.01 (d, J = 6.8 Hz, 3H); 13C NMR (CDCl3, 100 MHz): δ 185.8, 183.2, 159.8, 142.3, 141.0, 137.7, 136.5, 136.2, 135.1, 130.3, 125.5, 121.1, 119.9, 117.0, 96.0, 79.9, 56.7, 56.1, 31.6, 25.7, 25.2, 16.6; HRMS (ESI): calcd. for C22H23O5 (M + H)+ m/z 367.1545, found m/z 367.1532.
1 ethyl acetate/hexanes); mp = 208–211 °C; [α]22D = −34.0 (c 1.0, CHCl3); IR (CHCl3): 3020, 2928, 2884, 1669, 1587, 1468, 1448, 1269, 1217, 1087, 1030, 769 cm−1; 1H NMR (CDCl3, 400 MHz): δ 8.19 (d, J = 8.0 Hz, 1H), 7.85 (d, J = 6.8 Hz, 1H), 7.76 (d, J = 8.0 Hz, 1H), 7.69 (t, J = 8.0 Hz, 1H), 7.28 (d, J = 8.5 Hz, 1H), 4.85 (d, J = 6.8 Hz, 1H), 4.74 (d, J = 6.8 Hz, 1H), 4.40 (d, J = 4.8 Hz, 1H), 4.04 (s, 3H), 3.47 (s, 3H), 3.48–3.40 (m, 1H), 3.34–3.25 (m, 1H), 2.24–2.10 (m, 2H), 1.70–1.62 (m, 1H), 1.01 (d, J = 6.8 Hz, 3H); 13C NMR (CDCl3, 100 MHz): δ 185.8, 183.2, 159.8, 142.3, 141.0, 137.7, 136.5, 136.2, 135.1, 130.3, 125.5, 121.1, 119.9, 117.0, 96.0, 79.9, 56.7, 56.1, 31.6, 25.7, 25.2, 16.6; HRMS (ESI): calcd. for C22H23O5 (M + H)+ m/z 367.1545, found m/z 367.1532.
(3R,4S)-8-methoxy-4-(methoxymethoxy)-3-methyl-3,4-dihydrotetraphene-1,7,12(2H)-trione (20)
A solution of tetracycle 19 (200 mg, 0.54 mmol) in benzene (300 mL) was irradiated with a mercury lamp (125 W, Philips India) under a positive pressure of oxygen for 24 to 36 h. After the completion of reaction, the solvent was removed in vacuo to give crude product, which was subjected to purification by silica gel column chromatography (50% ethyl acetate in hexanes) to afford compound 20 (164 mg, 79%) as an orange solid; Rf = 0.35 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ethyl acetate/hexanes); mp = 209–213 °C; [α]22D = −99.0 (c 1.0, CHCl3); IR (CHCl3): 3016, 2961, 2933, 2846, 1702, 1672, 1588, 1471, 1444, 1300, 1272, 1223, 1149, 1035, 760 cm−1; 1H NMR (CDCl3, 400 MHz): δ 8.36 (d, J = 8.0 Hz, 1H), 7.79–7.69 (m, 3H), 7.32 (d, J = 8.2 Hz, 1H), 4.73 (d, J = 6.8 Hz, 1H), 4.65 (d, J = 6.8 Hz, 1H), 4.51 (d, J = 5.1 Hz, 1H), 4.04 (s, 3H), 3.38 (s, 3H), 3.25 (dd, J = 16.6, 6.6 Hz, 1H), 2.69–2.64 (m, 1H), 2.54 (dd, J = 16.6, 4.5 Hz, 1H), 1.09 (d, J = 7.0 Hz, 3H); 13C NMR (CDCl3, 100 MHz): δ 198.0, 184.1, 181.6, 161.0, 147.1, 137.5, 136.2, 135.6, 135.4, 134.3, 133.0, 129.8, 120.8, 119.9, 117.5, 95.7, 77.7, 56.7, 56.2, 43.5, 34.5, 18.7; HRMS (ESI): calcd. for C22H21O6 (M + H)+ m/z 381.1338, found m/z 381.1343.
1 ethyl acetate/hexanes); mp = 209–213 °C; [α]22D = −99.0 (c 1.0, CHCl3); IR (CHCl3): 3016, 2961, 2933, 2846, 1702, 1672, 1588, 1471, 1444, 1300, 1272, 1223, 1149, 1035, 760 cm−1; 1H NMR (CDCl3, 400 MHz): δ 8.36 (d, J = 8.0 Hz, 1H), 7.79–7.69 (m, 3H), 7.32 (d, J = 8.2 Hz, 1H), 4.73 (d, J = 6.8 Hz, 1H), 4.65 (d, J = 6.8 Hz, 1H), 4.51 (d, J = 5.1 Hz, 1H), 4.04 (s, 3H), 3.38 (s, 3H), 3.25 (dd, J = 16.6, 6.6 Hz, 1H), 2.69–2.64 (m, 1H), 2.54 (dd, J = 16.6, 4.5 Hz, 1H), 1.09 (d, J = 7.0 Hz, 3H); 13C NMR (CDCl3, 100 MHz): δ 198.0, 184.1, 181.6, 161.0, 147.1, 137.5, 136.2, 135.6, 135.4, 134.3, 133.0, 129.8, 120.8, 119.9, 117.5, 95.7, 77.7, 56.7, 56.2, 43.5, 34.5, 18.7; HRMS (ESI): calcd. for C22H21O6 (M + H)+ m/z 381.1338, found m/z 381.1343.
Fujianmycin A (5)
To a solution of tetracycle 20 (30 mg, 0.09 mmol) dissolved in CH2Cl2 (10 mL) was added anhy. AlCl3 (124 mg, 0.93 mmol, 10 equiv.) under inert atmosphere at 0 °C. The reaction mixture was stirred for 2 h at same temperature and quenched with ice water and extracted with CH2Cl2. Evaporation of the solvent and purification by silica gel column chromatography (40% ethyl acetate in hexanes) gave fujianmycin A 5 (23 mg, 92%) as a yellow solid; Rf = 0.4 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ethyl acetate/hexanes); mp = 185–188 °C; [α]22D = +11.8 (c 0.08, CHCl3); IR (KBr): 3444, 2957, 2928, 2846, 1697, 1668, 1583, 1467, 1437, 1273, 1223, 1108, 1063, 1034, 1017 cm−1; 1H NMR (CDCl3, 400 MHz): δ 12.27 (s, 1H), 8.38 (d, J = 8.2 Hz, 1H), 8.03 (d, J = 8.2 Hz, 1H), 7.69–7.64 (m, 2H), 7.27 (t, J = 6.7 Hz, 1H), 4.49 (d, J = 9.5 Hz, 1H), 3.13 (dd, J = 16.6, 6.2 Hz, 1H), 2.58 (dd, J = 16.6, 10.5 Hz, 1H), 2.43–2.31 (m, 1H), 1.27 (d, J = 6.6 Hz, 3H); 13C NMR (CDCl3, 100 MHz): δ 197.8, 187.5, 182.9, 162.3, 151.9, 137.3, 135.5, 135.3, 135.1, 134.2, 130.6, 129.7, 124.0, 119.9, 115.6, 73.5, 44.9, 38.2, 18.4; HRMS (ESI): calcd. for C19H14O5Na (M + Na)+ m/z 345.0739, found m/z 345.0752.
1 ethyl acetate/hexanes); mp = 185–188 °C; [α]22D = +11.8 (c 0.08, CHCl3); IR (KBr): 3444, 2957, 2928, 2846, 1697, 1668, 1583, 1467, 1437, 1273, 1223, 1108, 1063, 1034, 1017 cm−1; 1H NMR (CDCl3, 400 MHz): δ 12.27 (s, 1H), 8.38 (d, J = 8.2 Hz, 1H), 8.03 (d, J = 8.2 Hz, 1H), 7.69–7.64 (m, 2H), 7.27 (t, J = 6.7 Hz, 1H), 4.49 (d, J = 9.5 Hz, 1H), 3.13 (dd, J = 16.6, 6.2 Hz, 1H), 2.58 (dd, J = 16.6, 10.5 Hz, 1H), 2.43–2.31 (m, 1H), 1.27 (d, J = 6.6 Hz, 3H); 13C NMR (CDCl3, 100 MHz): δ 197.8, 187.5, 182.9, 162.3, 151.9, 137.3, 135.5, 135.3, 135.1, 134.2, 130.6, 129.7, 124.0, 119.9, 115.6, 73.5, 44.9, 38.2, 18.4; HRMS (ESI): calcd. for C19H14O5Na (M + Na)+ m/z 345.0739, found m/z 345.0752.
Rubiginone A2 (3)
To a stirred solution of MOM ether 20 (50 mg, 0.13 mmol) in MeOH (5 mL) at 0 °C was added dropwise AcCl (0.1 mL, 10 equiv.) and the reaction mixture was stirred for 12 h at room temperature. The reaction mixture was quenched with water, and extracted with CH2Cl2 (3 × 10 mL). The organic layer was washed with brine, dried over Na2SO4, and concentrated under vacuo. The crude compound was purified by silica gel column chromatography (60% ethyl acetate in hexanes) to afford rubiginone A2 3 (39.3 mg, 89%) as an orange solid; Rf = 0.25 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ethyl acetate/hexanes); mp = (decomp) > 218 °C; [α]22D = +62.0 (c 0.2, CHCl3); IR (CHCl3): 3413, 3059, 3017, 2927, 2854, 1669, 1590, 1437, 1303, 1272, 1217, 1175, 1119, 1067, 755 cm−1; 1H NMR (CDCl3, 400 MHz): δ 8.37 (d, J = 8.2 Hz, 1H), 7.99 (d, J = 8.2 Hz, 1H), 7.77 (d, J = 7.2 Hz, 1H), 7.73 (t, J = 8.0 Hz, 1H), 7.31 (d, J = 8.0 Hz, 1H), 4.50 (d, J = 9.3 Hz, 1H), 4.04 (s, 3H), 3.08 (dd, J = 16.4, 5.6 Hz, 1H), 2.59–2.52 (m, 1H), 2.41–2.36 (m, 1H), 1.26 (d, J = 6.4 Hz, 3H); 13C NMR (DMSO-d6, 100 MHz): δ 198.1, 184.4, 181.0, 160.0, 153.0, 137.2, 136.6, 135.2, 134.1, 134.0, 131.8, 129.7, 120.2, 119.1, 118.9, 72.1, 56.9, 44.8, 38.2, 18.6; HRMS (ESI): calcd. for C20H16O5Na (M + Na)+ m/z 359.0895, found m/z 359.0900.
1 ethyl acetate/hexanes); mp = (decomp) > 218 °C; [α]22D = +62.0 (c 0.2, CHCl3); IR (CHCl3): 3413, 3059, 3017, 2927, 2854, 1669, 1590, 1437, 1303, 1272, 1217, 1175, 1119, 1067, 755 cm−1; 1H NMR (CDCl3, 400 MHz): δ 8.37 (d, J = 8.2 Hz, 1H), 7.99 (d, J = 8.2 Hz, 1H), 7.77 (d, J = 7.2 Hz, 1H), 7.73 (t, J = 8.0 Hz, 1H), 7.31 (d, J = 8.0 Hz, 1H), 4.50 (d, J = 9.3 Hz, 1H), 4.04 (s, 3H), 3.08 (dd, J = 16.4, 5.6 Hz, 1H), 2.59–2.52 (m, 1H), 2.41–2.36 (m, 1H), 1.26 (d, J = 6.4 Hz, 3H); 13C NMR (DMSO-d6, 100 MHz): δ 198.1, 184.4, 181.0, 160.0, 153.0, 137.2, 136.6, 135.2, 134.1, 134.0, 131.8, 129.7, 120.2, 119.1, 118.9, 72.1, 56.9, 44.8, 38.2, 18.6; HRMS (ESI): calcd. for C20H16O5Na (M + Na)+ m/z 359.0895, found m/z 359.0900.
Rubiginone C2 (4)
To a solution of rubiginone A2 3 (50 mg, 0.15 mmol) dissolved in CH2Cl2 (5 mL) were added isobutyryl chloride (23.8 μL, 0.22 mmol) and 4-dimethylaminopyridine (36.6 mg, 0.3 mmol) at room temperature. The reaction mixture was stirred for 24 h, hydrolyzed with water and extracted with CH2Cl2. After workup and purification by silica gel column chromatography (30% ethyl acetate in hexanes), rubiginone C2 4 was obtained as a yellow solid (52 mg, 86%); Rf = 0.5 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ethyl acetate/hexanes); mp = 215–218 °C; [α]22D = −51.2 (c 0.5, CHCl3); IR (CHCl3): 2972, 2928, 2868, 1742, 1664, 1588, 1471, 1303, 1267, 1242, 1187, 1148, 1027, 759 cm−1; 1H NMR (CDCl3, 400 MHz): δ 8.35 (d, J = 8.2 Hz, 1H), 7.77 (dd, J = 7.6, 1.2 Hz, 1H), 7.72 (t, J = 8.2 Hz, 1H), 7.60 (d, J = 8.2 Hz, 1H), 7.32 (dd, J = 8.2, 1.2 Hz, 1H), 5.85 (d, J = 7.3 Hz, 1H), 4.04 (s, 3H), 3.2–3.14 (m, 1H), 2.72–2.59 (m, 3H), 1.24 (t, J = 7.1 Hz, 6H), 1.13 (d, J = 6.4 Hz, 3H); 13C NMR (CDCl3, 100 MHz): δ 196.8, 184.1, 181.3, 176.5, 160.0, 146.0, 137.5, 136.1, 135.7, 134.9, 134.7, 131.5, 130.2, 120.6, 119.8, 117.5, 73.4, 56.6, 43.9, 35.2, 34.2, 19.1, 19,0, 18.1; HRMS (ESI): calcd. for C24H23O6 (M + H)+ m/z 407.1495, found m/z 407.1479.
1 ethyl acetate/hexanes); mp = 215–218 °C; [α]22D = −51.2 (c 0.5, CHCl3); IR (CHCl3): 2972, 2928, 2868, 1742, 1664, 1588, 1471, 1303, 1267, 1242, 1187, 1148, 1027, 759 cm−1; 1H NMR (CDCl3, 400 MHz): δ 8.35 (d, J = 8.2 Hz, 1H), 7.77 (dd, J = 7.6, 1.2 Hz, 1H), 7.72 (t, J = 8.2 Hz, 1H), 7.60 (d, J = 8.2 Hz, 1H), 7.32 (dd, J = 8.2, 1.2 Hz, 1H), 5.85 (d, J = 7.3 Hz, 1H), 4.04 (s, 3H), 3.2–3.14 (m, 1H), 2.72–2.59 (m, 3H), 1.24 (t, J = 7.1 Hz, 6H), 1.13 (d, J = 6.4 Hz, 3H); 13C NMR (CDCl3, 100 MHz): δ 196.8, 184.1, 181.3, 176.5, 160.0, 146.0, 137.5, 136.1, 135.7, 134.9, 134.7, 131.5, 130.2, 120.6, 119.8, 117.5, 73.4, 56.6, 43.9, 35.2, 34.2, 19.1, 19,0, 18.1; HRMS (ESI): calcd. for C24H23O6 (M + H)+ m/z 407.1495, found m/z 407.1479.
Acknowledgements
We thank DST, New Delhi and IIT Bombay for financial support and the use of spectral facilities. K.P.K. thanks DST for the award of the Swarnajayanti fellowship. D.G.V. thanks UGC, New Delhi for a fellowship.Notes and references
- (a) M. K. Kharel, P. Pahari, M. D. Shepherd, N. Tibrewal, S. E. Nybo, K. A. Shaaban and J. Rohr, Nat. Prod. Rep., 2012, 29, 264–325 RSC; (b) M. C. Carreño and A. Urbano, Synlett, 2005, 1–25 CrossRef PubMed; (c) K. Krohn and J. Rohr, Top. Curr. Chem., 1997, 188, 127–195 CrossRef CAS; (d) J. Rohr and R. Thiericke, Nat. Prod. Rep., 1992, 9, 103–137 RSC.
- (a) D.-S. Hsu and J.-Y. Huang, J. Org. Chem., 2012, 77, 2659–2666 CrossRef CAS PubMed; (b) Q. Chen, M. Mulzer, P. Shi, P. J. Beuning, G. W. Coates and G. A. O'Doherty, Org. Lett., 2011, 13, 6592–6595 CrossRef CAS PubMed; (c) X. Yang, B. Fu and B. Yu, J. Am. Chem. Soc., 2011, 133, 12433–12435 CrossRef CAS PubMed; (d) C. Kesenheimer, A. Kalogerakis, A. Meissner and U. Groth, Chem.–Eur. J., 2010, 16, 8805–8821 CrossRef CAS PubMed.
- Recent papers on Hauser annulations: (a) R. Karmakar and D. Mal, J. Org. Chem., 2012, 77, 10235–10248 CrossRef CAS PubMed; (b) X. Yang, B. Fu and B. Yu, J. Am. Chem. Soc., 2011, 133, 12433–12435 CrossRef CAS PubMed; (c) S. Ray, A. Patra and D. Mal, Tetrahedron, 2008, 64, 3253–3267 CrossRef CAS PubMed; (d) D. Mal and P. Pahari, Chem. Rev., 2007, 107, 1892–1918 CrossRef CAS PubMed; (e) D. Mal and S. Dey, Tetrahedron, 2006, 62, 9589–9602 CrossRef CAS PubMed.
- P. W. Schindler, Biotech-Forum, 1989, 2, 142 Search PubMed.
- (a) K. Krohn, A. Agocs and C. J. Bäuerlein, J. Carbohydr. Chem., 2003, 22, 579–592 CrossRef CAS PubMed; (b) G. B. Caygill, D. S. Larsen and S. Broker, J. Org. Chem., 2001, 66, 7427–7431 CrossRef CAS PubMed; (c) T. Rozek, J. H. Bowie, S. M. Pyke, B. W. Skelton and A. J. White, J. Chem. Soc., Perkin Trans. 1, 2001, 1826–1830 RSC; (d) G. A. Kraus, N. Zhang, A. Melekhov and J. H. Jensen, Synlett, 2001, 521–522 CAS; (e) K. Krohn, J. Michael and M. Zukowski, Tetrahedron, 2000, 56, 4753–4758 CrossRef CAS; (f) G. Matsuo, Y. Miki, M. Nakata, S. Matsumura and K. J. Toshima, J. Org. Chem., 1999, 64, 7101–7106 CrossRef CAS; (g) K. Krohn and J. Micheel, Tetrahedron, 1998, 54, 4827–4838 CrossRef CAS; (h) D. S. Larsen and M. D. O'Shea, J. Chem. Soc., Perkin Trans. 1, 1995, 1019–1028 RSC; (i) T. Matsumoto, T. Sohma, H. Yamaguchi, S. Murata and K. Suzuki, Tetrahedron, 1995, 51, 7347–7360 CrossRef CAS; (j) D. S. Larsen and M. D. O'Shea, Tetrahedron Lett., 1993, 34, 1373–1376 CrossRef CAS; (k) K. Krohn, F. Ballwanz and W. Baltus, Liebigs Ann. Chem., 1993, 1993, 911–913 CrossRef; (l) A. Guingant and M. M. Barreto, Tetrahedron Lett., 1987, 28, 3107–3110 CrossRef CAS.
- (a) K. Kim, V. A. Boyd, A. Sobti and G. A. Sulikowski, Isr. J. Chem., 1997, 37, 3–22 CrossRef CAS; (b) V. A. Boyd and G. A. Sulikowski, J. Am. Chem. Soc., 1995, 117, 8472–8473 CrossRef CAS; (c) K. Kim and G. A. Sulikowski, Angew. Chem., Int. Ed. Engl., 1995, 34, 2396–2398 CrossRef CAS; (d) V. A. Boyd, B. E. Drake and G. A. Sulikowski, J. Org. Chem., 1993, 58, 3191–3193 CrossRef CAS.
- K. Krohn, M. H. Sohrab and U. Flörke, Tetrahedron: Asymmetry, 2004, 15, 713–718 CrossRef CAS PubMed.
- J. Motoyoshiya, Y. Masue, G. Iwayama, S. Yoshioka, Y. Nishii and H. Aoyama, Synthesis, 2004, 2099–2102 CrossRef CAS PubMed.
- (a) M. C. Carreño, A. Urbano and C. Di Vitta, Chem.–Eur. J., 2000, 6, 906–913 CrossRef; (b) M. C. Carreño, A. Urbano and C. Di Vitta, Chem. Commun., 1999, 817–818 RSC; (c) M. C. Carreño, A. Urbano and C. Di Vitta, J. Org. Chem., 1998, 63, 8320–8330 CrossRef; (d) M. C. Carreño, A. Urbano and J. Fischer, Angew. Chem., Int. Ed. Engl., 1997, 36, 1621–1623 CrossRef.
- (a) J. S. Landells, D. S. Larsen and J. Simpson, Tetrahedron Lett., 2003, 44, 5193–5196 CrossRef CAS; (b) D. S. Larsen, M. D. O'Shea and S. Broker, Chem. Commun., 1996, 203–204 RSC; (c) D. S. Larsen and M. D. O'Shea, J. Org. Chem., 1996, 61, 5681–5683 CrossRef CAS.
- (a) M. C. Carreño, M. Ribagorda, À. Somoza and A. Urbano, Angew. Chem., Int. Ed., 2002, 41, 2755–2757 CrossRef; (b) M. C. Carreño, À. Somoza, M. Ribagorda and A. Urbano, Chem.–Eur. J., 2007, 13, 879–890 CrossRef PubMed.
- M. Oka, H. Kamei, Y. Hamagishi, K. Tomita, T. Miyaki, M. Konishi and T. Oki, J. Antibiot., 1990, 43, 967–976 CrossRef CAS.
- R. W. Richards and J. P. Wu, J. Antibiot., 1985, 38, 513–515 CrossRef.
- K. Kimura, F. Kanou, H. Koshino, M. Uramoto and M. Yoshihama, J. Antibiot., 1997, 43, 967–976 Search PubMed.
- K. Kimura, F. Kano, K. Kurosawa and M. Yoshihama, Jap. Patent, No. 06234693, 1994, Chem. Abstr. 1995, 122, 8155.
- M. Oka, M. Konishi and T. Oki, Tetrahedron Lett., 1990, 31, 7473–7474 CrossRef CAS.
- (a) D. G. Vanga and K. P. Kaliappan, Synlett, 2012, 23, 2931–2934 CrossRef CAS PubMed; (b) D. G. Vanga and K. P. Kaliappan, Eur. J. Org. Chem., 2012, 2250–2259 CrossRef CAS; (c) K. P. Kaliappan and V. Ravikumar, J. Org. Chem., 2007, 72, 6116–6126 CrossRef CAS PubMed; (d) K. P. Kaliappan and V. Ravikumar, Synlett, 2007, 977–979 CrossRef CAS PubMed.
- Y. Gao, R. M. Hanson, J. M. Klunder, S. Y. Ko, H. Masamune and K. B. Sharpless, J. Am. Chem. Soc., 1987, 109, 5165–5180 Search PubMed.
- E. J. Corey, E. J. Trybulski, L. S. Melvin, Jr, K. C. Nicolaou, J. A. Secrist, R. Lett, P. W. Sheldrake, J. R. Falck, D. J. Brunelle, M. F. Haslanger, S. Kim and S. Yoo, J. Am. Chem. Soc., 1978, 100, 4618–4620 CrossRef CAS.
- M. Balestra, M. D. Wittman and J. Kallmerten, Tetrahedron Lett., 1988, 29, 6905–6908 CrossRef CAS.
- M. Rosillo, L. Casarrubios, G. Domínguez and J. Pérez-Castells, Tetrahedron Lett., 2001, 42, 7029–7031 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra47775d |
| This journal is © The Royal Society of Chemistry 2014 |