Unexpected different chemoselectivity in the aerobic oxidation of methylated planar catechin and bent epicatechin derivatives catalysed by the Trametes villosa laccase/1-hydroxybenzotriazole system†
Roberta Bernini*a,
Fernanda Crisantea,
Patrizia Gentili*b,
Sergio Mentac,
Fabio Moranab and
Marco Pierinic
aDipartimento di Scienze e Tecnologie per l'Agricoltura, le Foreste, la Natura e l'Energia (DAFNE), Università degli Studi della Tuscia, Via S. Camillo De Lellis, 01100 Viterbo, Italy. E-mail: berninir@unitus.it; Fax: +39 0761 357242; Tel: +39 0761 357452
bDipartimento di Chimica and IMC-CNR Sezione Meccanismi di Reazione, Università degli Studi La Sapienza, P. le A. Moro 5, 00185 Roma, Italy. E-mail: patrizia.gentili@uniroma1.it; Fax: +39 06 49913692; Tel: +39 06 49913082
cDipartimento di Chimica e Tecnologia del Farmaco, Università degli Studi La Sapienza, P. le A. Moro 5, I-00185 Roma, Italy
First published on 14th January 2014
Abstract
Unreported methylated catechin and epicatechin derivatives 5 and 6 were synthesized by an oxa-Pictet-Spengler reaction. Catechin 5 shows the B and C rings coplanar because of the formation of a trans junction between the C ring and the newly generated six-term cycle D, in turn condensed to ring B. In contrast, epicatechin 6 presents a bent geometry due to the establishment of a cis junction between the C ring and the newly formed cycle D. The oxidation of compounds 5 and 6 in the presence of the Trametes villosa laccase/1-hydroxybenzotriazole (HBT) system was investigated under aerobic conditions in both a biphasic system and a reverse micelle. The unexpected different chemoselective oxidation at the benzylic position of catechin and epicatechin derivatives 5 and 6 has been rationalized using a molecular modelling approach. These results demonstrate that the Trametes villosa laccase/HBT system represents a useful tool to functionalize the C-2 or C-4 position of phenolic compounds depending on the structural features.
Introduction
Laccases (benzenediol: oxygen oxidoreductase, EC 1.10.3.2) belong to a small group of enzymes known as the blue copper oxidases. These enzymes contain four catalytic copper atoms distributed in three redox sites. Originally described in plants,1 laccases can also be found in fungi and bacteria.2 Fungal laccases have higher redox potentials than bacterial or plant laccases, and their action appears to be relevant in nature, namely in the degradation of lignin – a finding with important implications for the field of biotechnology. Laccases are highly regarded as environmentally friendly catalysts in oxidation reactions.Being biodegradable and able to perform oxidations under mild experimental conditions, their use presents several advantages. Laccases can catalyse the monoelectronic oxidation of both organic compounds3 and inorganic ions4 with the simultaneous reduction of molecular dioxygen to water without the intermediate production of hydrogen peroxide. Laccases' potential use in oxidation reactions can be increased by combination with low molecular weight compounds termed mediators. These compounds enable these enzymes to oxidize substrates with a redox potential above 1.3 V (e.g. benzylic alcohols and ethers) by means of an indirect way where the active species is the oxidized form of the mediator.5
Mediators can act as an “electron shuttle”: once oxidized by laccases and converted to more or less stable radicals, they diffuse far away from the enzymatic pocket and, by mechanisms different from the enzymatic one, enable the oxidation of target compounds that in principle are not substrates of laccases because of their large size or high redox potential (Scheme 1).6
(+)-Catechin 1 and (−)-epicatechin 2 are representative members of flavonoids, a naturally occurring class of plant phenolic compounds widely distributed in foods and beverages, mainly in green and black tea (Fig. 1).7 Their biological and pharmacological effects, in particular radical scavenger activity, are well known. In the last few years, a synthetic catechin analogue in which the chroman and catechol moieties of 1 are coplanar by formation of a bridge between the 3-OH group on ring C and C-6′ on ring B has been identified as a novel and efficient antioxidant with reduced pro-oxidant activity potentially useful for the prevention and treatment of free radical-associated diseases.8
The enzymatic oxidations of polyphenols, and in particular of catechins, are widely described in the literature, being implicated in numerous biological processes in the vegetal world.9 In contrast, only a few articles have reported on the oxidative enzyme-catalysed chemistry of catechin derivatives for synthetic purposes.10 This lack of literature data is mainly due to the complexity of the reaction mixtures and the corresponding difficulties in isolating final oxidation products that are generally dimeric, oligomeric and polymeric compounds.
Taking into consideration our recent results regarding the oxidation of methylated catechins 3 and 4 with Trametes villosa laccase using 1-hydroxybenzotriazole (HBT) as mediator11 and as part of our research devoted to the selective oxidation of natural compounds in order to obtain fine chemicals and bioactive compounds,12 we projected the synthesis of new taxifolin-like compounds from methylated planar catechin 5 and methylated bent epicatechin derivative 6. Methylated catechin derivative 5 shows the B and C rings coplanar because of the formation of a trans junction between the C ring and the newly generated six-term cycle D, in turn condensed to ring B according to the synthetic catechin analogue reported by Fukuhara (Fig. 1).8 Contrarily, due to the establishment of a cis junction between the C ring and the newly formed cycle D, in its two more stable conformations modelled by DFT calculations (see below), methylated epicatechin derivative 6 presents bent geometry. The unexpected observed different chemoselectivity of oxidation of methylated planar catechin 5 and bent epicatechin 6 has been rationalized using a molecular modelling approach.
Results and discussion
1. Synthetic aspects
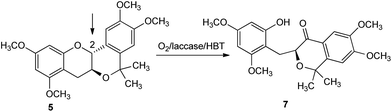
Catechin and epicatechin derivatives 5 and 6 were synthesized via an oxa-Pictet-Spengler reaction from the corresponding tetramethylated catechin 3 and epicatechin 4 in acetone using BF3–Et2O as Lewis acid (Scheme 2) according to Fukuhara's protocol.8,13Next, we focused our attention on their behaviour in air and in the presence of Trametes villosa laccase/HBT as the catalytic system. Based on our previous study on the oxidation of catechins 3 and 4,11 we initially chose aqueous sodium acetate buffer/tetrahydrofuran as the reaction medium. Unfortunately, despite the retained activity of the enzyme in this medium, methylated planar catechin 5 was not soluble. Therefore, owing to inactivation of the enzyme when the content of water-miscible organic solvent in the system increases14 and in an effort to use laccase in the presence of an organic solvent, we decided to perform the oxidation of catechin derivative 5 by the Trametes villosa laccase/HBT system in two types of reaction medium: (i) an aqueous sodium acetate buffer/ethyl acetate biphasic system15 and (ii) a sodium dioctylsulfosuccinate salt (AOT) reverse micelle system in isooctane/chloroform.16,17 In both these reaction environments, after 24 h, the laccase/HBT oxidation of methylated planar catechin 5 afforded exclusively compound 7 in 82% and 79% yields, respectively (Scheme 3; Table 1, entries 1 and 2), deriving from oxidation at the C-2 position.
In contrast, methylated bent epicatechin 6 was soluble in aqueous sodium acetate buffer/tetrahydrofuran, and after 50 h it afforded the oxidation products 8 and 9 in 64% and 18% yields, respectively (Scheme 4; Table 1, entry 3). Both products derive from oxidation at the C-4 position; compound 9 is a product of over-oxidation of the aromatic A ring.
The oxidation reactions conducted in the biphasic system and in the reverse micelle produced the same products 8 and 9 after only 24 h (Scheme 4; Table 1, entries 4 and 5). As far as we know, this is the first description of an oxidation reaction catalysed by the laccase/HBT system in either a biphasic system or a reverse micelle environment for synthetic purposes. Of note, the oxidation of methylated planar catechin derivative 5 and bent epicatechin 6 in these reaction media indicates that HBT acts as a “shuttle” connecting the organic phase, where substrates 5 and 6 are soluble, and the aqueous phase, where laccase retains its catalytic activity (Scheme 5). The use of these reaction media offers the additional advantage that further laccase addition during the oxidation is not necessary, as is required for the aqueous sodium acetate buffer/tetrahydrofuran medium (see Experimental section).11
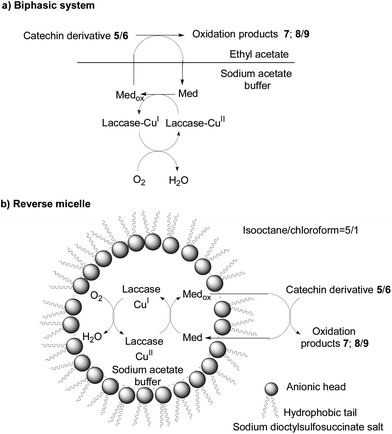 | ||
| Scheme 5 Oxidation of catechin derivatives 5 and 6 in (a) the biphasic system and (b) the reverse micelle. | ||
The experimental data evidence that oxidation of methylated bent epicatechin 6 by the laccase/HBT system leads to C-4 functionalization, in accordance with our previous results obtained by oxidation of methylated catechins 3 and 4.11 Unexpectedly, methylated planar catechin 5 is oxidized on the C-2 position. These results demonstrate the synthetic potentiality of the oxidative procedure in flavonoid chemistry and, in general, medicinal chemistry in order to produce a rearranged core structure or a ketone derivative depending on the structural features.
2. Mechanistic aspects
To shed light on such an intriguing finding, we undertook studies to investigate more precisely the origin of this different chemoselectivity. As described in the literature regarding the oxidation of a benzylic alcohol or ether derivatives by the laccase/HBT system, the key step is the abstraction of a hydrogen atom from the benzylic position by the oxidized form of the mediator, the benzotriazole-N-oxyl (BTNO) radical (Scheme 6).6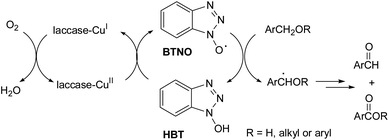 | ||
| Scheme 6 Catalytic cycle of the laccase/HBT system in the oxidation of a benzylic alcohol or ether derivatives. | ||
Indeed, determination of the oxidation potential by cyclic voltammetry in acetonitrile containing tetrabutylammonium tetrafluoroboride as supporting electrolyte indicated that methylated catechin and epicatechin derivatives 5 and 6 were irreversibly oxidized at a potential above 1.65 V (Table 2), thus confirming that they are resistant to monoelectronic oxidation and cannot be oxidized by Trametes villosa laccase (E° 0.79 V/NHE)18 directly.
| Sub | Ep (V, vs. NHE)a | kH (M−1 s−1)b | Sub.conf.c | BDEC–H (kJ mol−1)d | |||
|---|---|---|---|---|---|---|---|
| HFe | DFT2//HFf | DFTg | DFT2//DFTh | ||||
| a [Substrate]: 2 mM, at 500 mV s−1 in CH3CN containing Bu4NBF4 (0.1 M).b Rate constants obtained spectrophotometrically at 25 °C in CH3CN; initial condition: [BTNO] = 0.5 mM, [substrate] = 5–40 mM; determination in triplicate.c Calculated conformations of substrate.d From the following equation: BDEC–H = ΔH°f(R) + ΔH°f(H) – ΔH°f(R–H); ΔH°f(H) = 218.0 kJ mol−1.21e HF/3-21G.f B3LYP/6-311+G**//HF/3-21G.g B3LYP/6-31G*.h B3LYP/6-311+G**//B3LYP/6-31 G*.i Number of equivalent H atoms. | |||||||
| 5C-2 (1i) | 1.75 | 61 ± 1 | 5C-2 | 259.6 | 355.7 | 360.7 | 359.6 |
| 5C-4 (2i) | 5C-4 | 291.4 | 380.1 | 385.3 | 381.9 | ||
| 6C-2 (1i) | 1.69 | 56 ± 1 | 6aC-2 | 244.6 | 350.6 | 354.5 | 355.2 |
| 6C-4 (2i) | 6aC-4 | 289.4 | 370.4 | 378.9 | 373.2 | ||
| 6bC-2 | 268.7 | 358.6 | |||||
| 6bC-4 | 281.3 | 375.4 | |||||
3. Rationalization of the observed chemoselectivity
Estimations of BDEC–H values for H-abstraction from the C-2 or C-4 position of each compound 5 and 6 (one representative conformation for 5 and two for 6, hereafter denoted as 6a and 6b) were performed by quantum chemical calculations based on the hybrid Hartree-Fock (HF)/Density Functional Theory (DFT) method (for details see ESI†). Similar to what was previously observed for the parent structures 3 and 4,11 the achieved BDEC–H values (Table 2) indicated for both planar and bent catechin tetramethyl ethers 5 and 6 a more advantageous H-abstraction from carbon C-2 (359.6 and 355.2 kJ mol−1, respectively) than from C-4 (381.9 and 373.2 kJ mol−1, respectively). Moreover, the values attributed to the C-2 radical formation were found to be even smaller than those calculated elsewhere for 3 and 4 (377.4 and 367.4 kJ mol−1, respectively).11 However, such findings can only explain the C-2 functionalization observed in the oxidation of the planar catechin 5, but do not justify the reactivity manifested by compound 6 that led to the formation of 8 and 9 (i.e. the reactivity at carbon C-4). Again, taking inspiration from our previous study involving, amongst others, catechins 3 and 4,11 we theoretically evaluated the possibility that the observed C-4 reactivity of 6, assessed as thermodynamically disadvantageous, may originate from kinetic factors. For this purpose, four relevant transition states (TS) simulating H-abstraction from either the C-2 or C-4 position of 5 and 6a were also modelled (i.e. the structures hereafter denoted as TS_5C-2-BTNO, TS_5C-4-BTNO, TS_6aC-2-BTNO and TS_6aC-4-BTNO; Fig. S1;† details are given in the Experimental section and ESI†). From inspection of the achieved energetic results (Table S1 of ESI†), it is clear that methylated catechin and epicatechin derivatives 5 and 6 (in its more stable and greatly dominant 6a conformation) display a very different reactive behaviour towards oxidation. Indeed, catechin 5 shows an energetically more favourable and then faster H-abstraction from the C-2 carbon atom (ΔΔEC-2–C-45 = ETS_5C-2−BTNO − ETS_5C-4-BTNO = −12.30 kJ mol−1 from M06-2x calculations), whereas this occurs from the C-4 position for epicatechin 6 (ΔΔEC-2–C-46 = ETS_6aC-2-BTNO − ETS_6aC-4-BTNO = 10.85 kJ mol−1 from M06-2x calculations). This means that, at 25 °C and referring both the C-2 and C-4 HAT transition states of each catechin 5 or 6 to a relevant and common ground state adduct (hereafter denoted as 5:BTNO and 6a:BTNO, respectively), for compound 5 the generation of radical C-2 is assessed about 143 times faster than that of radical C-4, whereas for compound 6 it is estimated that radical C-2 is generated about 80 times slower than radical C-4. Interestingly, stable ground states of complexes 5:BTNO and 6a:BTNO displaying the BTNO oxygen virtually equidistant from both hydrogen atoms linked to carbons C-2 and C-4 (i.e. just geometries eligible to be considered as the common starting point leading to both transition states TS_XC-2-BTNO and TS_XC-4-BTNO, with X being equal to 5 or 6; Fig. 2 and ES1†) have indeed been obtained by suitable optimization at equilibrium geometry level of the structures TS_5C-2-BTNO and TS_6aC-4-BTNO.Therefore, in the case of epicatechin 6, the whole of the quoted findings resolutely agrees with the interpretation of a kinetic control of the process prevailing on the thermodynamic one, providing in this way a theoretical persuasive rationalization of the differential chemoselective oxidizability displayed by compounds 5 and 6. By visual analysis of the optimized structures obtained for TS_5C-2-BTNO, TS_5C-4-BTNO, TS_6aC-2-BTNO and TS_6aC-4-BTNO, a more direct relationship due to steric effects can be established between the observed chemoselectivity and the planar or bent structure of the considered catechins, as described in detail in the ESI.†
Conclusions
Unreported methylated planar catechin and bent epicatechin derivatives 5 and 6 were synthesized via an oxa-Pictet-Spengler reaction and then oxidized in air using Trametes villosa laccase/1-hydroxybenzotriazole as the catalytic system. The oxidations were performed in a biphasic system and in a reverse micelle and were determined to be chemoselective and efficient. To the best of our knowledge, this is the first time an exploitation of laccase/mediator in these reaction media for synthetic purposes has been reported. The different chemoselective oxidations of methylated planar catechin 5 (at the C-2 position) and bent epicatechin derivative 6 (at the C-4 position) were rationalized using a molecular modelling approach.Experimental
Materials and instruments
All chemicals were of the highest commercially available quality and were used as received. Crude laccase from Trametes villosa (Novozym 51002) was a gift from Novo Nordisk Co. and was free of lignin or manganese peroxidases. 1H NMR and 13C NMR spectra were recorded on a spectrometer 200 and 300 MHz using CDCl3 as solvent. All chemical shifts are expressed in parts per million (δ scale) and coupling constants are in Hertz (Hz). HPLC analysis was performed with a C18 column (150 mm × 4.6 mm × 5 µm) and a UV detector at λ = 280 nm. Samples were eluted at a flow rate of 1.0 mL min−1 with the following method: acetonitrile/water = 10/90 (0–10 min); linear gradient until a ratio of acetonitrile/water = 60/40 (10–25 min); acetonitrile/water = 60/40 (5 min); linear gradient until a ratio of acetonitrile/water = 80/20 (30–35 min) and then acetonitrile/water = 80/20 (5 min). High-resolution mass spectra (HRMS) were performed with an Electrospray Ionization Time of Flight Micromass spectrometer. Kinetic studies were performed with a stopped flow instrument interfaced to a diode array spectrophometer having a thermostated cuvette holder.Purification of laccase
Laccase from a strain of Trametes villosa (viz. Poliporus pinsitus) (Novo Nordisk Biotech) was employed. It was purified by ion-exchange chromatography on a Q-Sepharose column (pre-equilibrated with 10 mM Tris–HCl buffer at pH 7.5, 1 mS) by elution with a mixture of 10 mM Tris–HCl and 0.2 M NaCl (linear gradient 1–70 mM NaCl); laccase fractions having an absorption ratio A280/A610 of 20–30 were considered sufficiently pure.22 The collected fractions were concentrated by dialysis in cellulose membrane tubing (Sigma) against poly(ethylene glycol) to a final activity of 5277 U mL−1, as determined spectrophotometrically by the standard assay with ABTS (where 1 U is the number of µmol of ABTS oxidized by 1 mL of laccase solution per min).23Synthesis of catechin and epicatechin derivatives
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), methylated catechin 3 and 4 were isolated and characterized by spectroscopic analysis.
1), methylated catechin 3 and 4 were isolated and characterized by spectroscopic analysis.
Methylated catechin 3. Yellow solid (339 mg, 98%), mp 142–144 °C (from acetone/hexane). Spectroscopic data were identical with those in the literature.24
Methylated epicatechin 4. Yellow solid (329 mg, 95%), mp 144–146 °C (from acetone/hexane). Spectroscopic data were identical with those in the literature.24
Methylated planar catechin 5. White power (817 mg, 73%), mp: 136–138 °C (from acetone/hexane). Anal. Found: C, 68.2; H, 6.8; O: 25.0. Calcd. for C22H26O6: C, 68.4; H, 6.8; O, 24.8%. 1H-NMR (CDCl3): δ 1.57 (6H, s, 2 × CH3), 2.56 (1H, dd, J1 = 16 Hz, J2 = 10 Hz, 4-H), 3.07 (1H, dd, J1 = 16 Hz, J2 = 6.0 Hz, 4-H), 3.80 (7H, bs, 2 × OCH3, 3-H), 3.89 (3H, s, OCH3), 3.96 (3H, s, OCH3), 4.63 (1H, d, J = 8.0 Hz, 2-H), 6.12 (1H, s, 8-H), 6.20 (1H, s, 6-H), 6.56 (1H, s, 5′-H), 7.17 (1H, s, 2′-H). 13C-NMR (CDCl3): δ 26.9, 28.1, 31.6, 55.4, 55.5, 56.0, 56.1, 66.3, 73.3, 75.8, 91.9, 93.2, 102.5, 107.7, 108.0, 125.0, 135.1, 147.9, 148.5, 155.3, 158.9, 159.6.
Methylated bent epicatechin 6. White power (459 mg, 41%), mp 146–148 °C (from diethyl ether/hexane). Anal. Found: C, 68.1; H, 7.0; O, 24.9. Calcd. for C22H26O6: C, 68.4; H, 6.8: O, 24.8%. 1H-NMR (CDCl3): δ 1.51 (3H, s, CH3), 1.53 (3H, s, CH3), 2.89 (2H, d, J= 4.0 Hz, 4-H), 3.70 (3H, s, OCH3), 3.73 (3H, s, OCH3), 3.87 (3H, s, OCH3), 3.90 (3H, s, OCH3), 4.27 (1H, m, 3-H), 4.56 (1H, bs, 2-H), 6.05 (2H, m, 6, 8-H), 6.62 (1H, s, 5′-H), 6.86 (1H, s, 2′-H). 13C-NMR (CDCl3): δ 25.6, 27.6, 31.6, 55.3, 55.4, 56.0, 56.1, 63.3, 70.8, 75.5, 91.6, 93.2, 100.5, 108.3, 112.3, 123.9, 136.0, 147.8, 149.8, 155.0, 158.8, 159.3.
Oxidation of methylated planar catechin (5) and methylated bent epicatechin (6)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) buffered water (pH 4.7, 0.05 M NaOAc)/ethyl acetate. Compound 5 (or 6, 14 mg, 40 µmol) was solubilized in 2.0 mL of reaction medium, followed by the addition of 1-hydroxybenzotriazole HBT (5.4 mg, 40 µmol) and laccase (40 U). Reactions were monitored by TLC analysis. At the end of the experiment (24 h), the internal standard (1-methyl-6-methoxy-3,4-dihydro-1H-isochromen-7-ol, 7.8 mg, 40 µmol)11 was added. The organic phase was separated and the water phase was further extracted with ethyl acetate; the combined organic phases were washed with a saturated solution of NaCl and dried over Na2SO4. The yields of the oxidation reactions were determined by HPLC analysis with the internal standard method; suitable response factors were determined from authentic products. Pure samples of compound 7 (or 8 and 9) were obtained after chromatographic purifications using mixtures of hexane/ethyl acetate = 1/1 (v/v) and dichloromethane/methanol = 98/2 (v/v) and characterized by spectroscopic and analytical data.
1 (v/v) buffered water (pH 4.7, 0.05 M NaOAc)/ethyl acetate. Compound 5 (or 6, 14 mg, 40 µmol) was solubilized in 2.0 mL of reaction medium, followed by the addition of 1-hydroxybenzotriazole HBT (5.4 mg, 40 µmol) and laccase (40 U). Reactions were monitored by TLC analysis. At the end of the experiment (24 h), the internal standard (1-methyl-6-methoxy-3,4-dihydro-1H-isochromen-7-ol, 7.8 mg, 40 µmol)11 was added. The organic phase was separated and the water phase was further extracted with ethyl acetate; the combined organic phases were washed with a saturated solution of NaCl and dried over Na2SO4. The yields of the oxidation reactions were determined by HPLC analysis with the internal standard method; suitable response factors were determined from authentic products. Pure samples of compound 7 (or 8 and 9) were obtained after chromatographic purifications using mixtures of hexane/ethyl acetate = 1/1 (v/v) and dichloromethane/methanol = 98/2 (v/v) and characterized by spectroscopic and analytical data.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) buffered water (pH 4.7, 0.05 M sodium acetate)/THF. Compound 6 (14 mg, 40 µmol) was solubilized in 2.0 mL of reaction medium, followed by the addition of HBT (5.4 mg, 40 µmol) and laccase (40 U). Additional aliquots of laccase (40 U) were added every 3 h up until 280 U. Reaction was monitored by TLC analysis. At the end of the experiment (50 h), the internal standard (1-methyl-6-methoxy-3,4-dihydro-1H-isochromen-7-ol, 7.8 mg, 40 µmol)11 was added. The mixture was extracted with ethyl acetate; the organic phases were washed with a saturated solution of NaCl and dried over Na2SO4. The yield of the oxidation reaction was determined by HPLC analysis with the internal standard method; suitable response factors were determined from authentic products.
1 (v/v) buffered water (pH 4.7, 0.05 M sodium acetate)/THF. Compound 6 (14 mg, 40 µmol) was solubilized in 2.0 mL of reaction medium, followed by the addition of HBT (5.4 mg, 40 µmol) and laccase (40 U). Additional aliquots of laccase (40 U) were added every 3 h up until 280 U. Reaction was monitored by TLC analysis. At the end of the experiment (50 h), the internal standard (1-methyl-6-methoxy-3,4-dihydro-1H-isochromen-7-ol, 7.8 mg, 40 µmol)11 was added. The mixture was extracted with ethyl acetate; the organic phases were washed with a saturated solution of NaCl and dried over Na2SO4. The yield of the oxidation reaction was determined by HPLC analysis with the internal standard method; suitable response factors were determined from authentic products.
(S)-3-(2-Hydroxy-4,6-dimethoxybenzyl)-6,7-dimethoxy-1,1-dimethylisochroman-4-one 7. Yellow oil. 1H-NMR (CDCl3): δ 1.49 (6H, s, 2xCH3), 2.73 (1H, dd, J1 = 15 Hz, J2 = 9.0 Hz, CH2), 3.64 (1H, dd, J1 = 15 Hz, J2 = 3.0 Hz, CH2), 3.71 (3H, s, OCH3), 3.74 (3H, s, OCH3), 3.86 (3H, s, OCH3), 3.89 (3H, s, OCH3), 4.44 (1H, dd, J1 = 9.0 Hz, J2 = 3.0 Hz, 3-H), 6.02 (1H, d, J = 2.0 Hz, 5′-H), 6.11 (1H, d, J = 2.0 Hz, 3′-H), 6.52 (1H, s, 8-H), 7.41 (1H, s, 5-H), 8.33 (1H, s, OH). 13C-NMR (CDCl3): δ 24.9, 26.3, 29.7, 55.2, 55.7, 56.1, 56.2, 75.6, 76.4, 91.3, 94.5, 101.9, 105.8, 108.2, 131.9, 143.9, 148.4, 154.3, 157.6, 159.0, 160.1, 193.8. HRMS (ESI-TOF) m/z: [M + Na]+ Found 425.1576. Calcd. for C22H26O7Na 425.1590.
(6aS,12aR)-2,3,8,10-Tetramethoxy-5,5-dimethyl-6a,12a-dihydroisochromeno[4,3-b]chromen-7(5H)-one 8. Yellow oil. 1H-NMR (CDCl3): δ 1.58 (6H, s, 2xCH3), 3.81 (3H, s, OCH3), 3.89 (3H, s, OCH3), 3.91 (3H, s, OCH3), 3.94 (3H, s, OCH3), 4.15 (1H, d, J = 2.0 Hz, 6a-H), 4.99 (1H, d, J = 2.0 Hz, 12a-H), 6.08 (1H, d, J = 3.0 Hz, 11-H), 6.12 (1H, d, J = 3.0 Hz, 9-H), 6.64 (1H, s, 4-H), 6.89 (1H, s, 1-H). 13C-NMR (CDCl3): δ 27.8, 31.3, 55.9, 56.3, 56.4, 56.5, 71.5, 73.9, 76.4, 93.5, 93.6, 105.0, 108.3, 112.4, 120.7, 136.6, 148.4, 150.7, 163.3, 165.0, 166.7, 186.0. HRMS (ESI-TOF) m/z: [M + Na]+ Found 423.1420. Calcd. for C22H24O7Na 423.1457.
(6aR,12aR)-7-Hydroxy-2,3,10-trimethoxy-5,5-dimethyl-6a,7-dihydroisochromeno[4,3-b]chromene-8,11(5H,12aH)-dione 9. Yellow oil. 1H-NMR (CDCl3): δ 1.54 (3H, s, CH3), 1.58 (3H, s, CH3), 3.76 (3H, s, OCH3), 3.88 (3H, s, OCH3), 3.89 (3H, s, OCH3), 4.17 (1H, d, J = 2.0 Hz, 12a-H), 4.32 (1H, dd, J1 = 2.0 Hz, J2 = 1.0 Hz, 6a-H), 4.43 (1H, d, J = 1.0 Hz, 7-H), 5.72 (1H, s, 9-H), 6.57 (1H, s, 4-H), 6.75 (1H, s, 1-H). 13C-NMR (CDCl3): δ 27.8, 31.3, 56.3, 56.4, 57.0, 61.4, 62.8, 64.1, 76.4, 92.5, 107.3, 107.9, 112.9, 121.8, 127.5, 128.2, 136.1, 148.5, 150.2, 167.4, 193.6. HRMS (ESI-TOF) m/z: [M + Na]+ Found 425.1212. Calcd. for C21H22O8Na 425.1226.
Kinetic procedure
BTNO radical was generated in acetonitrile in a thermostated quartz cuvette (optical path = 1 cm) by adding a 0.5 mM solution of CAN to a 0.5 mM solution of HBT. A broad absorption band developed immediately (8 ms) in the λ = 350–600 nm region (λmax at 474 nm, ε = 1840 M−1 cm−1). The rate of decay was unaffected by the use of degassed acetonitrile. Rate constants of H-abstraction from catechin derivatives 5 and 6 were determined at 25 °C in acetonitrile by following the decrease of the absorbance at λ = 474 nm of BTNO. The initial concentrations of the substrate were in the 5 × 10−3 to 40 × 10−3 M range to enable a pseudo-first-order treatment of the kinetic data. A first order exponential was a good fit for plots of (At − A°) vs. time over more than three half-lives; the rate constants (k0) were obtained. From a plot of four to five k0 vs. [substrate]0 data pairs, the second-order rate constant of H-abstraction (kH) was obtained.Cyclic voltammetry
The electrochemical equipment consisted of a computer-controlled in-house potentiostat with a Vernier Software Multi Purpose Laboratory Interface (MPLI) program for Windows. The three electrodes consisted of a glassy-carbon disk (diameter of 1 mm) working electrode, an Ag/AgCl/KCl 3 M reference electrode (E° vs. NHE = E°(vs. Ag/AgCl) + 0.221), and a Pt auxiliary electrode (1 cm2). All scans were obtained at room temperature. The cyclic voltammetry scans of 2.0 mM of compounds 5 or 6 in acetonitrile solution containing 0.1 M Bu4NBF4 as supporting electrolyte, were run at a rate of 0.5 V s−1.Computational methods
All calculations were performed with software packages running on a PC equipped with Quad-Core AMD Opteron(tm) Processor 2376, 2.30-GHz (2 processors), 16 GB of RAM and OS 64-bit Windows 7 Ultimate. All optimizations of geometries of ground states, radicals, and transition states were performed by the computer program SPARTAN 08 (Wavefunction, Inc., Irvine, CA, USA). All details regarding the performed calculations are reported in ESI.†25References
- (a) S. Berthet, J. Thevenin, D. Baratiny, N. Demont-Caulet, I. Debeaujon, P. Bidzinski, J.-C. Leple, R. Huis, S. Hawkins, L.-D. Gomez, C. Lapiere and L. Jouanin, Adv. Bot. Res., 2012, 61, 145–172 CAS; (b) B. Gavnholt and K. Larsen, Physiol. Plant., 2002, 116, 273–280 CrossRef CAS.
- (a) N. Santhanam, J. M. Vivanco, S. R. Decker and K. F. Reardon, Trends Biotechnol., 2011, 29, 480–489 CrossRef CAS PubMed , and references cited therein; (b) U. N. Dwivedi, P. Singh, V. P. Pandey and A. Kumar, J. Mol. Catal. B: Enzym., 2011, 68, 117–128 CrossRef CAS PubMed; (c) P. Sharma, R. Goel and N. Capalash, World J. Microbiol. Biotechnol., 2007, 23, 823–832 CrossRef CAS.
- (a) J. Polak and A. Jarosz-Wilkolazka, Biotechnol. Prog., 2012, 28, 93–102 CrossRef CAS PubMed , and references cited therein; (b) P. Baldrian, K. Murugesan and Y.-S. Chang, Microb. Biotechnol., 2012, 5, 318–332 CrossRef PubMed; (c) F. Xu, T. Damhus, S. Danielsen and L. H. Ostergaard, in Modern Biooxidation: Enzymes, Reactions and Applications, ed. R. D. Schmid and V. B.Urlacher, Wiley-VCH Verlag GmbH & Co. KGaA, 2007, pp. 43–75, and references cited therein Search PubMed; (d) S. Witayakran and A. J. Ragauskas, Adv. Synth. Catal., 2009, 351, 1187–1209 CrossRef CAS.
- (a) M. Seki, J. Oikawa, T. Taguchi, T. Ohnuki, Y. Muramatsu, K. Sakamoto and S. Amachi, Environ. Sci. Technol., 2013, 47, 390–397 CrossRef CAS PubMed; (b) D. Schlosser and C. Hofer, Appl. Environ. Microbiol., 2002, 68, 3514–3521 CrossRef CAS; (c) J. Li-Cai, H. Pei-Zhi, J. Chang-Hua and D. Zi-Rong, Wuhan Univ. J. Nat. Sci., 2000, 5, 351–356 CrossRef; (d) C. Hofer and D. Schlosser, FEBS Lett., 1999, 451, 186–190 CrossRef CAS.
- (a) A. I. Cañas and S. Camarero, Biotechnol. Adv., 2010, 28, 694–705 CrossRef PubMed , and references cited therein; (b) R. Bourbonnais and M. G. Paice, FEBS Lett., 1990, 267, 99–102 CrossRef CAS; (c) H. P. Call and I. Mucke, J. Biotechnol., 1997, 53, 163–202 CrossRef CAS.
- (a) P. Astolfi, P. Brandi, C. Galli, P. Gentili, M. F. Gerini, L. Greci and O. Lanzalunga, New J. Chem., 2005, 29, 1–10 RSC , and references cited therein; (b) C. Galli and P. Gentili, J. Phys. Org. Chem., 2004, 17, 973–977 CrossRef CAS , and references cited therein; (c) F. d'Acunzo and C. Galli, Eur. J. Biochem., 2003, 270, 3634–3640 CrossRef CAS; (d) Y. Zhao and D. G. Truhlar, Theor. Chem. Acc., 2008, 120, 215–241 CrossRef CAS.
- (a) W. Y. Feng, Curr. Drug Metab., 2006, 7, 755–809 CrossRef CAS; (b) N. Khan and H. Mukhtar, Life Sci., 2007, 519–533 CrossRef CAS PubMed; (c) J. D. Lambert, S. Sang and C. S. Yang, Mol. Pharm., 2007, 4, 819–825 CrossRef CAS PubMed.
- (a) K. Fukuhara, I. Nakanishi, T. Shimada, K. Ohkubo, K. Miyazaki, W. Hakamata, S. Urano, T. Ozawa, H. Okuda, N. Miyata, N. Ikota and S. Fukuzumi, Chem. Res. Toxicol., 2003, 16, 81–86 CrossRef CAS PubMed; (b) I. Nakanishi, K. Ohkubo, K. Miyazaki, W. Hakamata, S. Urano, T. Ozawa, H. Okuda, S. Fukuzumi, N. Ikota and K. Fukuhara, Chem. Res. Toxicol., 2004, 17, 26–31 CrossRef CAS PubMed.
- (a) V. Cheynier, C. Osse and J. Rigaud, J. Food Sci., 1988, 53, 1729–1731 CrossRef CAS; (b) Y. Hirose, H. Yamaoka and M. Nakayama, J. Am. Oil Chem. Soc., 1991, 68, 131–132 CrossRef CAS; (c) T. Tanaka, M. Takahashi, H. Hagino, S.-I. Nudejima, H. Usui, T. Fujii and M. Taniguchi, Chem. Eng. Sci., 2009, 65, 569–573 CrossRef PubMed; (d) D. Areskogh, J. Li, P. Nousiainen, G. Gellerstedt, J. Sipilae and G. Henriksson, Holzforschung, 2010, 64, 21–34 CrossRef CAS; (e) S. Ghidouche, N.-E. Es-Safi and P.-H. Ducrot, Tetrahedron Lett., 2008, 49, 619–623 CrossRef CAS PubMed , and references therein.
- (a) M. Kurisawa, J. E. Chung, H. Uyama and S. Kobayashi, Macromol. Biosci., 2003, 3, 758–764 CrossRef CAS; (b) H. Shibuya, A. Agusta, K. Ohashi, S. Maehara and P. Simanjuntak, Chem. Pharm. Bull., 2005, 53, 866–867 CrossRef CAS; (c) A. Agusta, S. Maehara, K. Ohashi, P. Simanjuntak and H. Shibuya, Chem. Pharm. Bull., 2005, 53, 1565–1569 CrossRef CAS.
- R. Bernini, F. Crisante, P. Gentili, F. Morana, M. Pierini and M. Piras, J. Org. Chem., 2011, 76, 820–832 CrossRef CAS PubMed.
- (a) M. Barontini, R. Bernini, F. Crisante and G. Fabrizi, Tetrahedron, 2010, 66, 6047–6053 CrossRef CAS PubMed; (b) R. Bernini, F. Crisante, G. Fabrizi and P. Gentili, Curr. Org. Chem., 2012, 16, 1051–1057 CrossRef CAS; (c) R. Bernini, G. Fabrizi, L. Pouységu, D. Deffieux and S. Quideau, Curr. Org. Synth., 2012, 9, 650–669 CrossRef CAS; (d) R. Bernini, F. Crisante, M. Barontini, D. Tofani, V. Balducci and A. Gambacorta, J. Agric. Food Chem., 2012, 60, 7408–7416 CrossRef CAS PubMed.
- K. Fukuhara, I. Nakanishi, H. Kansui, E. Sugiyama, M. Kimura, T. Shimada, S. Urano, K. Yamaguchi and N. Miyata, J. Am. Chem. Soc., 2002, 124, 5952 CrossRef CAS PubMed.
- (a) Y. Y. Wan, R. Lu, L. Xiao, Y.-M. Du, T. Miyakoshi, C.-L. Chen, C. J. Knill and J. F. Kennedy, Int. J. Biol. Macromol., 2010, 47, 488–495 CrossRef CAS PubMed; (b) J. Rodakiewicz-Nowak and A. Jarosz-Wilkolazka, J. Mol. Catal. B: Enzym., 2007, 44, 53–59 CrossRef CAS PubMed , and references cited therein; (c) F. d'Acunzo, A. M. Barreca and C. Galli, J. Mol. Catal. B: Enzym., 2004, 31, 25–30 CrossRef CAS PubMed , and references cited therein.
- (a) C. Navarra, P. Gavezzotti, D. Monti, W. Panzeri and S. Riva, J. Mol. Catal. B: Enzym., 2012, 84, 115–120 CrossRef CAS PubMed , and references cited therein; (b) O. E. Adelakun, T. I. Kudanga, I. R. Green, M. Le Roes-Hill and S. G. Burton, Process Biochem., 2012, 47, 1926–1932 CrossRef CAS PubMed , and references cited therein; (c) C. Navarra, C. Goodwin, S. Burton, B. Danieli and S. Riva, J. Mol. Catal. B: Enzym., 2010, 65, 52–57 CrossRef CAS PubMed , and references cited therein.
- (a) N. L. Klyachko, N. G. Bogdanove, A. V. Levashov and K. Martinek, Collect. Czech. Chem. Commun., 1992, 57, 625–640 CrossRef CAS; (b) A. V. Pshezhetskii, N. L. Klyachko, A. V. Levashov and K. Martinek, Biocatalysis, 1990, 4, 185–198 CrossRef CAS.
- (a) J. Michizoe, H. Ichinose, N. Kamiya, T. Maruyama and M. Goto, J. Biosci. Bioeng., 2005, 99, 642–647 CrossRef CAS PubMed; (b) S. Ozakazi, J. Michizoe, M. Goto, S. Furusaki, H. Wariishi and H. Tanaka, Enzyme Microb. Technol., 2002, 31, 227–232 CrossRef; (c) J. Michizoe, M. Goto and S. Furusaki, J. Biosci. Bioeng., 2001, 92, 67–71 CAS.
- F. Xu, A. E. Palmer, D. S. Yaver, R. M. Berka, G. A. Gambetta, S. H. Brown and E. I. Solomon, J. Biol. Chem., 1999, 274, 12372–12375 CrossRef CAS PubMed.
- P. Brandi, C. Galli and P. Gentili, J. Org. Chem., 2005, 70, 9521–9528 CrossRef CAS PubMed.
- (a) D. Griller, J. A. Howard, P. R. Marriott and J. C. Scaiano, J. Am. Chem. Soc., 1981, 103, 619–624 CrossRef CAS; (b) A. L. J. Beckwith and C. J. Easton, J. Am. Chem. Soc., 1981, 103, 615–619 CrossRef CAS; (c) V. Malatesta and J. C. Scaiano, J. Org. Chem., 1982, 47, 1455–1459 CrossRef CAS.
- CRC Handbook of Chemistry and Physics, ed. D. R. Lide, CRC Press Boca Raton, FL., 74 edn, 1993 Search PubMed.
- F. Xu, Biochemistry, 1996, 35, 7608–7614 CrossRef CAS PubMed.
- B. S. Wolfenden and R. L. Willson, J. Chem. Soc., Perkin Trans. 2, 1982, 805–812 RSC.
- (a) H. Liu, Y. Yamazaki, T. Sasaki, M. Uchida, H. Tanaka and S. Oka, Phytochemistry, 1999, 51, 297–308 CrossRef; (b) K. Hori, T. Satake, Y. Saiki, T. Murakami and C.-M. Chen, Chem. Pharm. Bull., 1988, 36, 4301–4306 CrossRef CAS.
- (a) O. Tishchenko and D. G. Truhlar, J. Phys. Chem. Lett., 2012, 3, 2834–2839 CrossRef CAS; (b) I. Hermans, P. Jacobs and J. Peeters, Phys. Chem. Chem. Phys., 2008, 10, 1125–1132 RSC.
Footnote |
| † Electronic supplementary information (ESI): 1H-NMR and 13C-NMR spectra of compounds 5, 6, 7, 8 and 9; computational methods and Cartesian coordinates of all the optimized molecular structures. See DOI: 10.1039/c3ra47753c |
| This journal is © The Royal Society of Chemistry 2014 |