A dual-ionic liquid microemulsion system for the selective isolation of hemoglobin
Quan-Xing Maoa,
Hui Wanga,
Yang Shub,
Xu-Wei Chen*a and
Jian-Hua Wang*ac
aResearch Center for Analytical Sciences, Colleges of Sciences, Northeastern University, Box 332, Shenyang, China. E-mail: chenxw@mail.neu.edu.cn; jianhuajrz@mail.neu.edu.cn; Fax: +86 24 83676698; Tel: +86 24 83688944
bInstitute of Biological Technology, Colleges of Life and Health Sciences, Northeastern University, Shenyang, China
cCollaborative Innovation Center of Chemical Science and Engineering, Tianjin, 300071, China
First published on 14th January 2014
Abstract
A novel dual-ionic liquid (IL) microemulsion system is developed with IL 1-decyl-3-methylimidazolium bromide (DmimBr) as the surfactant and IL 1-butyl-3-methylimidazolium hexaflourophosphate (BmimPF6) as a substitute for organic solvent. The phase diagram of this dual-IL microemulsion system clearly shows the formation of a single phase within a wide range of BmimPF6 (1.76–98.3%, wt) and in the presence of an appropriate amount of DmimBr and water. The microemulsion is characterized by means of FT-IR spectra, dynamic light scattering and molecular probe. The dual-IL microemulsion system has been demonstrated to be effective for the extraction of proteins, and exhibited an obvious improvement in extraction efficiency for hemoglobin in comparison with pure BmimPF6. When 100 μL microemulsion is used to extract 100 μg mL−1 of proteins in an equal volume of sample solution, high selective extraction of hemoglobin (Hb) has been observed at pH 5. This might be attributed to the coordination interaction between the heme group of Hb and the imidazolium cationic moiety in the ionic liquids. Hb transferred into the microemulsion can be readily recovered by back extraction with Britton–Robinson buffer at pH 12, giving rise to a recovery of 55.6%. The dual-IL microemulsion system is practically applied to the isolation of Hb from human whole blood and the SDS-PAGE indicates that hemoglobin has been selectively isolated from human blood in the presence of co-existing protein species.
Introduction
Microemulsion is a kind of thermodynamically stable, isotropic and optically transparent system consisting of water, oil and a surfactant. It is widely used in many fields including extraction and separation of various species.1 The water core in microemulsion facilitates effective extraction of proteins2–6 and it helps to maintain the activity of proteins during the extraction process.7 The use of organic solvents in conventional microemulsion systems causes serious adverse effects on protein structure and property, which find limited application in protein separation.Microemulsion system containing ionic liquid (IL) is a promising alternative to conventional microemulsion with toxic organic solvents. IL exhibits favorable biocompatibility due to its unique features of negligible vapor pressure, high chemical/thermal stability and nonflammability. In this respect, it provides green approaches for the extraction of proteins or other biomacromolecules.8–10 In addition the structure of IL can be readily designed to offer adjustable properties which bring large possibilities for the development of IL-based mocroemulsion systems for extracting the targets of interest. Recently, efforts have been dedicated to theoretical studies on microemulsion systems with ionic liquids,11–15 where conventional organic surfactants or co-surfactants are involved. From the view point of life science applications, the use of organics is highly unexpected. Thus, the development of novel IL-based microemulsion systems without organic solvents or surfactants are highly desired in the field of separation sciences.16,17
Long chain ionic liquids have been investigated as novel surfactants due to the amphiphilic nature of their cations or anions. The emphases of studies are focused on the aggregation behaviour of IL in water.18–23 Specific self-assembled structures of ILs can be generated by altering the parameters of alkyl chain length, the cationic structure and the anionic nature. These IL-based surfactants are effective as templates for the synthesis of different nanostructured materials.24–26
In this study, ionic liquids 1-butyl-3-methylimidazolium hexaflourophosphate (BmimPF6) and 1-decyl-3-methylimidazolium bromine (DmimBr) have been used as the oil phase and surfactant respectively for the formation of a water/1-decyl-3-methylimidazolium bromine (DmimBr)/1-butyl-3-methylimidazolium hexaflourophosphate (BmimPF6) (water/DmimBr/BmimPF6) dual-IL microemulsion system. This novel microemulsion system shows high selectivity to hemoglobin (Hb) in the extraction of protein species. The extraction efficiency of Hb with this dual-IL microemulsion system is greatly improved when compared to that by using pure ionic liquid BmimPF6.
Experimental
Instrumentation
Protein quantification and spectral measurement of methyl orange in dual-IL microemulsion are performed by a U-3900 UV-vis spectrophotometer (Hitachi High Technologies, Japan). Dynamic light scattering (DLS) measurement is performed with a nano ZS-90 particle size analyzer (Malvern Instruments, United Kingdom). FT-IR spectra are obtained by a Nicolet-6700 spectrophotometer (Thermo Instruments Inc., USA) within a range of 400 cm−1 to 4000 cm−1. A PB-10 pH meter (Beijing Sartorius Instruments Co., Ltd., China) is used for pH measurement.Reagents
Hemoglobin (Hb, H2625, 95%), bovine serum albumin (BSA, A1933, >98%), cytochrome c (cyt-c, 30398, >95%), tris(hydroxymethyl)aminomethane (Tris, T1378, >99.9%) are the products of Sigma-Aldrich (St. Louis, USA). Protein Marker is obtained from Takara Biotechnology (Dalian, China). 1-decyl-3-methylimidazolium bromine (DmimBr) and 1-butyl-3-methylimidazolium hexaflourophosphate (BmimPF6) are purchased from Cheng Jie Chemicals. (Shanghai, China). Human blood sample is donated by a volunteer and collected by the Hospital of Northeastern University (Shenyang, China). Other reagents including methyl orange, NaOH, H3PO4, CH3COOH, H3BO3, HCl, H2NCONH2 and NaCl are obtained from Sinopharm Chemical Reagent (Shanghai, China).All reagents used are at least of analytical reagent grade unless otherwise specified. Deionized water (DI water) of 18 MΩ cm−1 is used throughout.
Characterization of the dual-IL microemulsion system
Phase diagram of the dual-IL miocroemulsion system is characterized at 25 °C by visually observing transition from clear transparent solution to turbid solution or on the contrary.14,15,27,28 0.87 g of BmimPF6 and 0.05 g of H2O are mixed and mechanically vibrated for 20 min in a 50 mL tube. The mixture is centrifuged at 8000 rpm for 5 min for phase separation. Appropriate amount of DmimBr is afterwards added and the mixture is mechanically vibrated for 20 min for thorough mixing. Then, the mixture is centrifugated at 8000 rpm for 5 min for the collection of reagents. A homogeneous transparent phase is observed after a series of the above successive operations, where the first critical point is achieved. Subsequently, certain amount of water is added to determine the second critical point at which the microemulsion turns turbid or separates to tow phases after same operations as performed for determining the first point. The other critical points are determined by following exactly the same manner as those for the first and the second ones. The mass of the individual component for forming the microemulsion is measured at each critical point, and the phase diagram of the dual-IL microemulsion is thus developed based on these data.The aggregate sizes in the dual-IL microemulsion systems with different R values (molar ratio of BmimPF6/DmimBr) are measured at 25 °C by using a nano ZS-90 particle size analyzer (Malvern Instruments, United Kingdom). The dual-IL microemulsions are prepared by mixing 0.7 g of water, 0.4 g of DmimBr with different amount of BmimPF6, e.g., 0.02, 0.04, 0.06 and 0.08 g. The mixture is mechanically vibrated for 20 min and centrifugated at 8000 rpm for 5 min.
The dual-IL microemulsions with a fixed R value of 5.7 and various amount of H2O (0, 0.015, 0.035 and 0.070 g) are prepared. Their FT-IR spectra are measured by using a Nicolet-6700 spectrometer (Thermo Instruments Inc., United States) within a range of 400–4000 cm−1. The dual-IL microemulsions with methyl orange (ranging from 1.6 to 7.6 wt%) are prepared by adding certain volume of methyl orange solution (1.50 × 10−4 mol L−1) into 100 μL of mixture of BmimPF6/DmimBr (0.8 g/0.15 g, R is 5.7). The content of water in the microemulsion is approximately equal to that of the added MO solution, e.g., ranging from 1.6 to 7.6 wt%. UV-vis spectra of methyl orange in dual-IL microemulsions are then recorded with a U-3900 UV-vis spectrophotometer (Hitachi High Technologies, Japan) within 370–505 nm.
Extraction of proteins by the dual-IL microemulsion
For this purpose, a dual-IL microemulsion system consisting of 0.8 g of BmimPF6, 0.15 g of DmimBr and 0.015 g of water is prepared. 100 μL of protein solution is mixed with an equal volume of the dual-IL microemulsion. The mixture is then mechanically vibrated for 20 min to facilitate the extraction of protein into the microemulsion. After centrifugation at 8000 rpm for 5 min, the supernatant is collected and the amount of residual protein is determined by spectrophotometry at 406 nm for Hb, 409 nm for cyt-c and 596 nm for BSA. Extraction efficiency (E) is derived according to the following equation, where C0 and Cr are the protein concentrations in the original sample solution and the collected supernatant after extraction, V0 and Vr are the volumes of original sample and the supernatant. | (1) |
In order to recover the extracted protein, the dual-IL microemulsion after extraction is mixed with 100 μL of Britton–Robinson buffer solution at pH 12. The mixture is vibrated for 30 min followed by centrifugation at 8000 rpm for 5 min. The supernatant containing recovered protein is collected for the ensuing investigations.
Results and discussion
Characteristics of the dual-IL microemulsion system
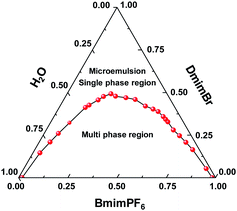
The amphipathicity of DmimBr, which originates from the long hydrophobic alkyl chain and the hydrophilic imidazole group, makes it an appropriate alternative for traditional surfactants in the formation of microemulsion. On the other hand, the excellent hydrophobicity of BmimPF6 makes it a suitable oil phase. Fig. 1 illustrates the phase diagram of the water/DmimBr/BmimPF6 microemulsion system derived at 25 °C. It is obvious that the phase diagram is divided into two parts, i.e., the single-phase (microemulsion) region and the multi-phase region. A stable and transparent dual-IL microemulsion is formed when mixing appropriate amount of water, DmimBr and BmimPF6 according to their mass ratios as illustrated in the single-phase region.The size of aggregate in the dual-IL microemulsion is determined by using dynamic light scattering technique. The results summarized in Table 1 illustrate an increase of aggregate size with the increase of R value within a certain range. The close dependence of aggregate size on the amount of the oil-like ionic liquid BmimPF6 clearly indicates that the DmimBr aggregates swallow or encapsulate BmimPF6 molecules and the aggregates expand with more BmimPF6 entering into its cavity. These results are well consistent with those observed in previously reported IL/W microemulsion systems.27,28
| Composition of the dual-IL microemulsion | R value | Aggregate size (nm) | ||
|---|---|---|---|---|
| BmimPF6 (wt%) | DmimBr (wt%) | H2O (wt%) | ||
| 1.79 | 35.71 | 62.50 | 0.05 | 1.12 ± 0.10 |
| 3.51 | 35.09 | 61.40 | 0.11 | 1.35 ± 0.15 |
| 5.17 | 34.48 | 60.35 | 0.16 | 1.69 ± 0.12 |
| 6.78 | 33.90 | 59.32 | 0.21 | 1.98 ± 0.04 |
The microenvironment of the dual-IL microemulsion can be reflected by its structural information which is closely related to the states of water molecules solubilized in the microemulsion. The various chemical states of water molecules can be translated into vibrational absorptions in infrared spectra.29,30 There are three distinct forms of water molecules in microemulsion system, i.e., trapped water, bound water and free water.31 Trapped water usually disperses along the hydrophobic alkyl-chain of surfactants. It cannot form hydrogen bond with surroundings and is characterized by high frequency absorption at ca. 3600 cm−1, attributing to the O–H stretching vibration. Bound water is the one combined with the polar head of surfactants via hydrogen bond, contributing to a stretching vibration adsorption of O–H at ca. 3400 cm−1. Free water molecules in microemulsion system are similar to those in bulk water, located in the core of surfactant aggregate. The formation of hydrogen bond among free water molecules shifts the O–H stretching vibration adsorption to a lower band at ca. 3220 ± 20 cm−1.29
Fig. 2 shows FT-IR spectra of the dual-IL microemulsion with different water content. The adsorption bands at 3596.5 cm−1 and 3407.7 cm−1 well suggest the existence of trapped water and bound water in the formed microemulsion. It is worth mentioning that no adsorption bands are observed within 3220 ± 20 cm−1, indicating that there exist no free water molecules in the dual-IL microemulsion. The observations herein are in accordance with what reported in earlier literatures27,32 that water molecules are prone to stay in the palisade layer of microemulsion or bond to the polar heads of hydrophobic surfactants, rather than enter into the core of the formed aggregates.
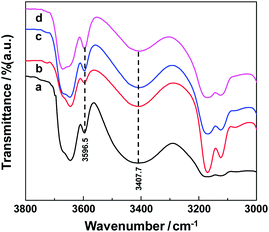 | ||
| Fig. 2 FT-IR spectra of the H2O/DmimBr/BmimPF6 microemulsion with different percentage of water at a fixed R value of 5.7. (a) 0 wt%, (b) 1.5 wt%, (c) 3.6 wt%, (d) 6.8 wt%. | ||
Methyl orange is sensitive to the surrounding polarity33 and thus it is usually used as a probe to obtain the inner structural information of microemulsions by monitoring the polarity changes.34–36 Fig. 3 shows the absorption spectra of methyl orange in the dual-IL microemulsion. The absorption maximum (λmax) of MO is red-shifted from 425.8 nm to 432.4 nm with the increase of MO content from 1.6 to 5.4 wt%. Further increase of the MO content results in no shift of λmax and it remains constant at 432.4 nm. This can be explained by the fact that methyl orange is not soluble in BmimPF6 and therefore it can just stay at the palisade layer or enter into the core of the aggregates.31 The polarity of the palisade layer microenvironment is enhanced by increasing water molecules until saturation is reached, at which water molecules begin to bond with imidazole group or enter into the core of the aggregates and thus no change of polarity is recorded.34,37,38 The above results indicate the formation of W/IL microemulsion.
 | ||
| Fig. 3 UV-vis spectra of methyl orange in the dual-IL microemulsion as a function of the added amount of methyl orange at a fixed R value of 5.7. | ||
Extraction of proteins by the water/DmimBr/BmimPF6 microemulsion
Hemoglobin (Hb), bovine serum albumin (BSA) and cytochrome c (cyt-c) are used as representatives of acidic, neutral and basic proteins to investigate their extraction behaviors by the water/DmimBr/BmimPF6 dual-IL microemulsion system and by pure ionic liquid BmimPF6, the results are illustrated in Fig. 4a–e. It indicates that the extraction of proteins is highly pH-dependent. The microemulsion gives rise to a complete extraction of hemoglobin in the range of pH 3–5, followed by a decline of the extraction efficiency when further increasing the pH value. At pH < 7, an extraction efficiency as high as ca. 70% could be maintained. At the same time, virtually no extraction of BSA and cyt-c is observed at pH 6.Coordination interaction between the nitrogen atom and the iron atom has been reported previously.39,40 Hb is a kind of heme proteins, in which the sixth vacant coordinating position of iron atom in heme group is available to bind with other small molecules or ligands. Therefore, the coordination interaction between the heme group and the cationic moiety of imidazole IL has been demonstrated to be the main driving force to facilitate the transfer of heme-proteins into IL phase.41 It is worth mentioning that with the increase of pH value, the quaternary structure of Hb converts from tight T form (deoxy form) to loose R form (oxy form). The latter form of Hb prefers to coordinate with oxygen instead of nitrogen.42,43 Thus the competition in coordination interaction from oxygen leads to the decrease of Hb extraction efficiency with the increase of pH value.
Unlike Hb, the coordinating positions of iron atom of heme group in cyt-c are fully occupied by strong-field protein ligands, i.e., histidyl-18 and methionyl-80.44 Therefore there are no vacant coordinating sites for the binding of external molecules, and thus the extraction of cyt-c by the microemulsion is virtually not observed at all within the pH range investigated.
Considering that two imidazolium ILs are involved in the dual-IL microemulsion system, the extraction of Hb by pure BmimPF6 is investigated as a comparison, and the results are illustrated in Fig. 4d. It is obvious that for the case of pure BmimPF6 a similar trend is found for the variation of extraction efficiency of Hb as a function of pH value with respect to that by the dual-IL microemulsion system. Within the range of pH 3–6, an obvious improvement in extraction efficiency has been observed from <70% for pure BmimPF6 to ca. 100% for the microemulsion system. UV-vis spectra of Hb in different media are illustrated in Fig. 5, showing a maximum absorption at 406 nm in aqueous solution. The absorption is red-shifted to 411 nm in BmimPF6, while on the contrast, it is blue-shifted to 396 nm in both the dual-IL microemulsion and DmimBr aqueous solution. This observation indicates that there exist certain interaction between Hb and the DmimBr which facilitates Hb extraction into the ionic liquid phase. Thus the improvement on Hb extraction in the dual-IL microemulsion might be contributed by the introduction of long-chain imidazolium IL DmimBr, which offers additional imidazolium moiety to coordinate with the heme group.
 | ||
| Fig. 5 Uv-vis spectra of Hb (100 μg mL−1) in different environment (a: aqueous solution, b: dual-IL microemulsion, c: 5.35 × 10−2 mol L−1 DmimBr solution, d: BmimPF6). | ||
For the case of BSA, an extraction efficiency of ca. 76% is achieved at pH 3. While the increase of pH value causes a sharp drop on the extraction efficiency, and no extraction is observed at pH 6 or even higher. Further investigations have shown that the extraction behavior of BSA by the dual-IL microemulsion system is quite similar to that observed by pure BmimPF6, as illustrated in Fig. 4e. This clearly indicates that the hydrophilic IL DmimBr in the microemulsion system contributes nothing to the extraction of BSA. Further investigations are required to elucidate the mechanisms on this issue.
Selective extraction of Hb by the dual-IL microemulsion
The results in Fig. 4 indicated that selective extraction of hemoglobin by the dual-IL microemulsion in the presence of other protein species is feasible at pH 5–6. For this purpose, some key parameters governing the extraction process are thoroughly investigated. The stability of the microemulsion system and its extraction capacity for hemoglobin depend strongly on the composition of the microemulsion. Thus the effect of the mass of water, DmimBr and BmimPF6 are studied to obtain the best extraction efficiency under a fixed sample volume of 100 μL. The results indicate that the microemulsion is not stable in the extraction process when the mass of BmimPF6 is <0.25 g. A stable microemulsion system is obtained when adopting 0.8 g of BmimPF6. It is found that an increase of the extraction efficiency of Hb is achieved with the increase of the DmimBr mass up to 0.15 g, probably due to the increase of the DmimBr aggregates formed. Finally, 0.15 g DmimBr is used for the formation of the dual-IL microemulsion system. Control experiments of DmimBr/BmimPF6 and working microemulsions with different amount of water content have been performed. The results indicate that water content has no effect on the extraction efficiency. When considering that water is needed to form microemulsion but more water may induce phase transition, 0.015 g water is used to form the microemulsion.Considering that high concentrations of electrolytes are involved in biological samples with complex matrix components, the effect of ionic strength on the extraction efficiency is thus investigated. The results illustrate that at an ionic strength of as high as 5 mol L−1, no suppression effect is observed and quantitative extraction of 100 μg mL−1 Hb is achieved. This result also well suggests that electrostatic and/or hydrophobic interactions are not involved in the driving forces for the extraction of hemoglobin into the water/DmimBr/BmimPF6 microemulsion system. While electrostatic and/or hydrophobic interactions are usually regarded as the main driving forces for mass transferring in conventional single IL-based microemulsion systems.45
It is generally preferential to perform ensuing biological investigations in aqueous media after the extraction of protein from complex biological samples by the dual-IL microemulsion. The back-extraction of Hb from the microemulsion system by a series of reagents or solvents has been investigated. The results indicate that poor recoveries are obtained when Tris–HCl (pH 9.0), Na2HPO4–NaH2PO4 (pH 8.9), NaCl (2.0 mol L−1), urea (0.5–6.0 mol L−1) and imidazole (1.0 mol L−1) are used, due to the strong coordination interaction between heme group and cationic imidazolium moiety in the ILs. When we look back to the pH dependent extraction efficiency as illustrated in Fig. 4, an alkali B–R buffer might be a candidate for the back extraction of hemoglobin. On the other hand, B–R buffer is a commonly used solvent for the separation and detection of biomolecules.46,47 The results have shown an increase of the recovery for hemoglobin with the increase of pH value of the B–R buffer from 7–12, and a recovery of 55.6% is achieved at pH 12.
Isolation of Hb from human whole blood
The water/DmimBr/BmimPF6 microemulsion is used for the isolation of Hb from human whole blood which is donated by a healthy volunteer. The blood is anticoagulated with sodium citrate and then diluted to 100-fold with B–R buffer at pH 5. The supernatant is collected after centrifugation to perform extraction with the water/DmimBr/BmimPF6 microemulsion. The solutions after extraction and recovery are both collected for analysis by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) assay.The SDS-PAGE assay is conducted as described in literature.48 12% polyacrylamide separation gel and 5% polyacrylamide stacking gel are prepared in electrophoretic tank. The blood sample is then mixed with loading buffer (a mixture of 250 mmol L−1 Tris–HCl (pH 6.8), 10% (w/v) SDS, 0.5% (w/v) bromophenol blue, 50% (v/v) glycerine, and 5% (w/v) β-mercaptoethanol). The mixture is kept boiling for 5 min and then injected into a concave space of 5% polyacrylamide gel. A voltage of 90 V is taken in the stacking gel stage and 180 V is taken in the separation gel stage. The protein bands are visualized after staining with 0.2% Coomassie brilliant blue G250 for 1 h and decoloration with a solution consisting of 7.5% (v/v) acetic acid and 5% (v/v) methanol. The standard SDS-PAGE results are shown in Fig. 6.
A few protein bands are visualized for human whole blood in lane 2, indicating the existence of Hb, albumin, cyt-c and transferrin in the diluted human whole blood. After extraction, the majority of protein bands are still there except for the fact that the band at ca. 14.3 kDa becomes weaker (lane 3). After back extraction a single hemoglobin band in lane 4 is clearly observed, which is the same as that for the standard hemoglobin solution in lane 5, indicating a high purity of the recovered hemoglobin.
Conclusion
A dual-ionic liquid microemulsion system is prepared by mixing water, 1-decyl-3-methylimidazolium bromine (DmimBr) and 1-butyl-3-methylimidazolium hexaflourophosphate (BmimPF6). The microemulsion used for the purpose of extraction is a W/IL type, which exhibits specific selectivity for the extraction of hemoglobin in the presence of other protein species. This approach provides a promising alternative for the isolation of biomacromolecules from complex biological sample matrixes.Acknowledgements
Financial support from National Natural Science Foundation of China (21275027, 21235001, 21075013, 21105008), the Program of New Century Excellent Talents in University (NCET-11-0071) and Fundamental Research Funds for the Central Universities (N110705002, N110805001) are highly appreciated.Notes and references
- B. G. Mazi, H. Hamamci and S. R. Dungan, Food Chem., 2012, 132, 326 CrossRef CAS PubMed.
- D. P. Hong, S. S. Lee and R. Kuboi, J. Chromatogr. B: Biomed. Sci. Appl., 2000, 743, 203 CrossRef CAS.
- K. E. Nandini and N. K. Rastogi, Process Biochem., 2009, 44, 1172 CrossRef CAS PubMed.
- U. H. Hebbar, B. S. A. B. Hemavathi and K. S. M. S. Raghavarao, Food Bioprocess Technol., 2012, 5, 1010 CrossRef CAS.
- J. A. G. del Rio and D. G. Hayes, Biotechnol. Prog., 2011, 27, 1091 CrossRef PubMed.
- J. Xiao, J. Cai and X. Guo, Food Chem., 2013, 136, 1063 CrossRef CAS PubMed.
- H. Noritomi, S. Ito, N. Kojima, S. Kato and K. Nagahama, Colloid Polym. Sci., 2006, 284, 604 CAS.
- X. W. Chen, Q. X. Mao and J. H. Wang, Prog. Chem., 2013, 25, 661 CAS.
- S. N. Baker, T. M. McCleskey, S. Pandey and G. A. Baker, Chem. Commun., 2004, 940 RSC.
- K. Fujita, M. Forsyth, D. R. MacFarlane, R. W. Reid and G. D. Elliott, Biotechnol. Bioeng., 2006, 94, 2109 CrossRef PubMed.
- R. Rai, S. Pandey, S. N. Baker, S. Vora, K. Behera, G. A. Baker and S. Pandey, Chem. – Eur. J., 2012, 18, 12213 CrossRef CAS PubMed.
- J. H. Porada, M. Mansueto, S. Laschat and C. Stubenrauch, Soft Matter, 2011, 7, 6805 RSC.
- O. Zech, S. Thomaier, A. Kolodziejski, D. Touraud, I. Grillo and W. Kunz, Chem. – Eur. J., 2010, 16, 783 CrossRef CAS PubMed.
- V. G. Rao, S. Mandal, S. Ghosh, C. Banerjee and N. Sarkar, J. Phys. Chem. B, 2013, 117, 1480 CrossRef CAS PubMed.
- O. Rojas, B. Tiersch, S. Frasca, U. Wollenberger and J. Koetz, Colloids Surf., A, 2010, 369, 82 CrossRef CAS PubMed.
- T. L. Greaves and C. J. Drummond., Chem. Soc. Rev., 2008, 37, 1709 RSC.
- W. Kunz, T. Zemb and A. Harrar, Curr. Opin. Colloid Interface Sci., 2012, 17, 205 CrossRef CAS PubMed.
- H. Y. Wang, Q. Q. Feng, J. J. Wang and H. C. Zhang, J. Phys. Chem. B, 2010, 114, 1380 CrossRef CAS PubMed.
- Y. Zhao, S. J. Gao, J. J. Wang and J. M. Tang, J. Phys. Chem. B, 2008, 112, 2031 CrossRef CAS PubMed.
- H. Y. Wang, J. J. Wang, S. B. Zhang and X. P. Xuan, J. Phys. Chem. B, 2008, 112, 16682 CrossRef CAS PubMed.
- M. Blesic, M. H. Marques, N. V. Plechkova, K. R. Seddon, L. P. N. Rebelo and A. Lopes, Green Chem., 2007, 9, 481 RSC.
- J. Bowers, C. P. Butts, P. J. Martin and M. C. V. Gutierrez, Langmuir, 2004, 20, 2191 CrossRef CAS.
- J. J. Wang, L. M. Zhang, H. Y. Wang and C. Z. Wu, J. Phys. Chem. B, 2011, 115, 4955 CrossRef CAS PubMed.
- A. M. Dattelbaum, S. N. Baker and G. A. Baker, Chem. Commun., 2005, 939 RSC.
- T. W. Wang, H. Kaper, M. Antonietti and B. Smarsly, Langmuir, 2007, 23, 1489 CrossRef CAS PubMed.
- Z. H. Li, Z. Jia, Y. X. Luan and T. C. Mu, Curr. Opin. Solid State Mater. Sci., 2008, 12, 1 CrossRef CAS PubMed.
- A. Safavi, N. Maleki and F. Farjami, Colloids Surf., A, 2010, 355, 61 CrossRef CAS PubMed.
- V. G. Rao, S. Ghosh, C. Ghatak, S. Mandal, U. Brahmachari and N. Sarkar, J. Phys. Chem. B, 2012, 116, 2850 CrossRef CAS PubMed.
- N. Li, Q. Cao, Y. A. Gao, J. Zhang, L. Q. Zheng, X. T. Bai, B. Dong, Z. Li, M. W. Zhao and L. Yu, ChemPhysChem, 2007, 8, 2211 CrossRef CAS PubMed.
- Y. A. Gao, N. Li, L. Q. Zheng, X. Y. Zhao, J. Zhang, Q. Cao, M. W. Zhao, Z. Li and G. Y. Zhang, Chem. – Eur. J., 2007, 13, 2661 CrossRef CAS PubMed.
- N. Li, S. H. Zhang, H. C. Ma and L. Q. Zheng, Langmuir, 2010, 26, 9315 CrossRef CAS PubMed.
- Y. A. Gao, N. Li, L. Q. Zheng, X. T. Bai, L. Yu, X. Y. Zhao, J. Zhang, M. W. Zhao and Z. Li, J. Phys. Chem. B, 2007, 111, 2506 CrossRef CAS PubMed.
- Y. A. Gao, W. Z. Wu, B. X. Han, G. Z. Li, J. W. Chen and W. G. Hou, Fluid Phase Equilib., 2004, 226, 301 CrossRef CAS PubMed.
- V. G. Rao, S. Mandal, S. Ghosh, C. Banerjee and N. Sarkar, J. Phys. Chem. B, 2012, 116, 13868 CrossRef CAS PubMed.
- S. K. Metha, K. Kaur, Bhawna and K. K. Bhasin, Colloids Surf., A, 2009, 339, 217 CrossRef PubMed.
- L. M. Qi and J. M. Ma, J. Colloid Interface Sci., 1998, 197, 36 CrossRef CAS.
- Y. A. Gao, S. B. Han, B. X. Han, G. Z. Li, D. Shen, Z. H. Li, J. M. Du, W. G. Hou and G. Y. Zhang, Langmuir, 2005, 21, 5681 CrossRef CAS PubMed.
- Y. A. Gao, N. Li, L. Q. Zheng, X. Y. Zhao, S. H. Zhang, B. X. Han, W. G. Hou and G. Z. Li, Green Chem., 2006, 8, 43 RSC.
- D. Barrick, Biochemistry, 1994, 33, 46546 CrossRef.
- J. Hirst, S. K. Wilcox, P. A. Williams, J. D. Blankenship, E. McRee and D. B. Goodin, Biochemistry, 2001, 40, 1265 CrossRef CAS PubMed.
- D. H. Cheng, X. W. Chen, Y. Shu and J. H. Wang, Talanta, 2008, 75, 1270 CrossRef CAS PubMed.
- S. De and A. Girigoswami, J. Colloid Interface Sci., 2006, 296, 324 CrossRef CAS PubMed.
- S. Bettati and A. Mozzarelli, J. Biol. Chem., 1997, 272, 32050 CrossRef CAS PubMed.
- K. Shimojo, N. Kamiya, F. Tani, H. Naganawa, Y. Naruta and M. Goto, Anal. Chem., 2006, 78, 7735 CrossRef CAS PubMed.
- Y. Shu, D. H. Cheng, X. W. Chen and J. H. Wang, Sep. Purif. Technol., 2008, 64, 154 CrossRef CAS PubMed.
- Z. G. Chen, G. L. Liu, M. H. Chen, Y. R. Peng and M. Y. Wu, Anal. Biochem., 2009, 384, 337 CrossRef CAS PubMed.
- W. W. Song, N. B. Li and H. Q. Luo, Anal. Biochem., 2012, 422, 1 CrossRef CAS PubMed.
- U. K. Laemmli, Nature, 1970, 227, 680 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |