Magnetic field assisted growth of highly dense α-Fe2O3 single crystal nanosheets and their application in water treatment
Abstract
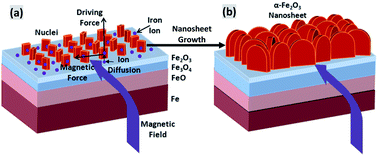
Highly dense 2D nanostructures are desirable in photocatalysis, water treatment and energy storage, due to their high surface to volume areas. This paper describes a novel approach combining thermal stress and magnetic force to generate highly dense α-Fe2O3 nanosheets on the surface of various iron substrates, including plates and powders. This technique involves the thermal oxidation of iron substrates on a hot plate with a magnetic field. The Lorentz force acting on the ions induced by the magnetic field facilitates the lateral growth of nanosheets. This effect results in a highly porous nanostructure consisting of dense 2D nanosheets with extremely large BET surface areas. The application of these nanosheets is explored in water treatment. Electron microscopic studies indicate that these nanosheets show a parabolic relation with time of thermal oxidation for the growth in the width direction. A comparison of heavy metal (As, Cr) ion adsorption of nanosheets and nanowires was also performed, which shows that nanosheets have a much better adsorption rate than nanowires.

 Please wait while we load your content...
Please wait while we load your content...