Intramolecular cyclization–decyclization of new sterically hindered diiminophenol. Synthesis and coordination abilities†
Abstract
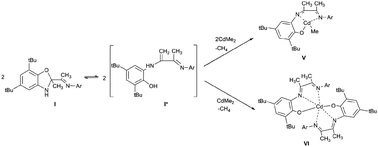
The new sterically hindered benzoxazole I was synthesized by the reaction of 3-(2,6-diisopropylphenylimino)butan-2-one and 2-amino-4,6-di-tert-butylphenol. It is shown that compound I is in equilibrium with the open enamine form in solution. The coordination abilities of I have been studied. The ligand I is shown to demonstrate either a neutral coordination type in complex with cadmium iodide or monoanionic type in cadmium complexes obtained by the interaction of I with Me2Cd.

 Please wait while we load your content...
Please wait while we load your content...