Characterization and phase transition study of a versatile molecular gel from a glucose-triazole-hydrogenated cardanol conjugate†
Abstract
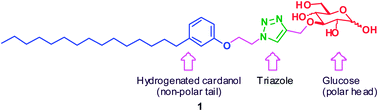
The synthesis and gelation properties of a structurally simple, renewable-resource-based glucose-triazole-hydrogenated cardanol conjugate (GTHCC) are reported. The conjugation of hydrogenated cardanol and glucose was done using a triazole linker employing copper(I) mediated acetylene azide cycloaddition ‘click’ chemistry in the key step. The GTHCC was found to form four different classes of gels; namely, (i) hydrogel from aqueous-protic solvents, (ii) organogel from non-polar solvents, (iii) gel from vegetable oil and (iv) gel from petroleum oil. In a water–methanol (1 : 1) mixture, the conjugate acted as a stable thermoreversible supergelator, even at a very low gelator concentration of 0.03% w/v. Unlike glycoside-based gels derived from hydrogenated cardanol and glucose, the GTHCC is stable under acidic as well as basic conditions, owing to the covalent linkages in the tether. The intrinsic fluorescence of the hydrogel was found to be sensitive towards a gel–sol transition.

 Please wait while we load your content...
Please wait while we load your content...