Architecture of rose and hollow marigold-like ZnIn2S4 flowers: structural, optical and photocatalytic study†
Abstract
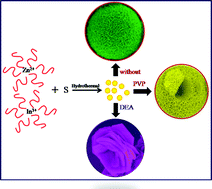
In the present investigation, a surfactant-assisted hydrothermal route has been employed to design self-assembled rose and hollow marigold-like ZnIn2S4 flowers. In the absence of the surfactant, uniform (∼3–5 μm) marigold-like flowers are observed. The self-alignment of the transparent petals (∼3–5 nm thick with a length of ∼25–100 nm) leads to the formation of hollow marigold-like flowers, for which a plausible growth mechanism has also been proposed. Moreover, DEA assisted ZnIn2S4 demonstrates a rose flower-like via self assembly of hexagonal nanoplates. Structural and optical characterization shows the existence of hexagonal structures with a band gap in the range of ∼2.4–2.6 eV. Considering the ideal band gap in the visible region, we have used such unique nanostructured self assemblies of ZnIn2S4 as photocatalysts and demonstrated visible light-driven photocatalytic production of clean hydrogen by toxic hydrogen sulphide, which is abundantly available as a waste gas from oil refineries (15–20%). We believe that continuous efforts in this direction may open up new insights into the design of controllable nanostructures and their potential applications in advanced fields.

 Please wait while we load your content...
Please wait while we load your content...