Built-up superhydrophobic composite membrane with carbon nanotubes for water desalination†
Bowu Zhang‡
a,
Lixia Liu‡bc,
Siyuan Xieac,
Fei Shen*b,
Hui Yanb,
Huanhuan Wubc,
Yinhua Wanb,
Ming Yua,
Hongjuan Maa,
Linfan Lia and
Jingye Li*a
aTMSR Research Center and CAS Key Laboratory of Nuclear Radiation and Nuclear Energy Techniques, Shanghai Institute of Applied Physics, Chinese Academy of Sciences, Shanghai 201800, P. R. China. E-mail: jingyeli@sinap.ac.cn; Tel: +86-21-39194505
bNational Key Lab of Biochemical Engineering, Institute of Process Engineering, Chinese Academy of Sciences, Beijing 100190, P. R. China. E-mail: fshen@ipe.ac.cn; Tel: +86-10-82544991
cUniversity of Chinese Academy of Sciences, Beijing 100049, P. R. China
First published on 21st February 2014
Abstract
Superhydrophobic porous composite membranes are successfully prepared by using poly(vinyl acetate) functionalized multi-walled carbon nanotubes and tested for water desalination under a direct contact membrane distillation (DCMD) method. The permeate flux of the composite membranes remains greater than 20 kg m−2 h−1 and the salt rejection greater than 99.5% when tested with 3.5% NaCl solution at 70 °C. The water contact angle of the composite membranes remains greater than 150° after DCMD testing for 2 hours.
As population growth and climate change continue, the scarcity of fresh water resources and the need for additional water supplies are becoming more and more critical in many countries, and are not limited to the arid regions of the world.1 Under this situation of an increasingly serious water crisis,2 desalination has attracted much attention from the scientific and industrial communities,3 and is believed to be one of the most promising and sustainable approaches to produce fresh water since about 97.5% of the water on Earth is saline water.4 Regarding state-of-the-art of water desalination, reverse osmosis (RO) and membrane distillation (MD) are the most interesting technologies in the water industry.5 Due to the limitation of osmotic pressure, a high water recovery coefficient and desalination ratio of the RO process are not attainable.6 MD is a thermally-driven membrane separation process, in which the saline water is separated into hot brine and cold distillate by a hydrophobic microporous membrane.7 The hydrophobic porous membrane acts as a physical barrier that prevents liquid penetration by surface tension force, but does not barricade vapor.8 Therefore, the fresh water obtained from the MD process could theoretically have 100% salt rejection. As a matter of fact, the common hydrophobic membrane is prone to wetting in the MD operation. We performed the direct contact membrane distillation (DCMD) process (Fig. S1†) using a commercial porous PVDF membrane with a pore size of 0.45 μm, and it was wetted after the test, in which the average water contact angle (CA) of this membrane decreased from 123.5° to 90.5° (Fig. S2a†). Gryta et al. reported that hydrophilic groups could sometimes be formed on a hydrophobic polymer membrane surface, which reduced its hydrophobicity and induced membrane wetting.9 Membrane wetting (or pore wetting) is the main obstacle in the application of the MD process, which results in a decline of the process efficiency.10 Our experimental results also show the salt rejection of the PVDF membrane decreases obviously after just 5 hours of DCMD operation (Fig. S2b†). That is because the wetted membrane would result in capillary condensation inside the pores of the membrane, and liquid penetration through the membrane.9 As such, it is very important for MD applications to study how to avoid or reduce membrane wetting.
Superhydrophobic surfaces exhibit both a high static water CA greater than 150° and a low water roll-off angle of less than 10°, which is known as the “Lotus Effect”.11 A superhydrophobic membrane surface can show improved water repellency compared to a hydrophobic one due to the existence of an extra air gap between the water drop and the surface.12 In other words, the superhydrophobicity can be expected to enhance the membrane’s overall performance in the MD process by maximizing the allowable pore sizes and minimizing pore wetting.13 Recently, functional porous membranes with superhydrophobicity have been designed and fabricated. Cho et al. prepared a superhydrophobic membrane, which consists of an elastic polyurethane fibrous matrix coated with polyaniline hairy nanostructures and polytetrafluoroethylene, and exhibits excellent anti-wettability and gas breathability.14 Zhang et al. successfully fabricated a superhydrophobic PVDF-based membrane with a CA of 156° for DCMD applications by spraying a mixture of polydimethylsiloxane and hydrophobic SiO2 nanoparticles on porous PVDF membranes, and after 5 h of immersion in boiling water, the modified membrane still had a superhydrophobic surface.13
Carbon nanotubes (CNT) possess high hydrophobicity, chemical inertness, nanoscale dimensions and a high aspect ratio, and have been used to build up superhydrophobic materials. For instance, a CNT-based filter with superhydrophobic and superoleophilic properties was fabricated for water/oil separation by CVD growth on stainless steel meshes.15,16 It indicates that CNT-based surfaces possess good water-repellence. Dumee et al. reported self-supporting CNT Bucky-papers which could be used for desalination in a DCMD setup with 99% salt rejection and a flux rate of ∼12 kg m−2 h−1.17 However, the delamination of this CNT membrane limits its performance (i.e. lifetime and permeability). Liu et al. found that CNT could impart hydrophobic properties to textiles, but could not endow them with a long-lasting hydrophobic surface.18 After CNT were modified with poly(butyl acrylate)18 and poly(4-azidophenylmethacrylate-co-methyl acrylate),19 stability in the superhydrophobicity of the textile surface was realized, due to the enhanced interaction between the textile and the CNTs with the assistance of the polymer chains on the CNT. Therefore, surface functionalization with hydrophobic polymer chains is a practical way to improve the stability of the superhydrophobicity of CNT composite membranes.
The radiation-induced graft polymerization technique, where high energy γ-rays can break the covalent bonds within the matrix randomly and induce the formation of new covalent bonds between the matrix and the grafted polymer chains,20 has been successfully applied to CNT functionalization.21 Enhanced stability be aided by the formation of the covalent bonds between the matrix and the grafted polymer chains, which has been demonstrated in the preparation of the laundering and abrasion durable superhydrophobic cotton fabrics in our previous study.22
Herein, we report our work on the use of polymer functionalized CNT to build up superhydrophobic composite membranes and their use in a DCMD setup for salt water desalination. Firstly, the pristine multi-walled carbon nanotubes (shortened as CNT herein) were functionalized with poly(vinyl acetate) (PVAc) via radiation-induced graft polymerization.23 The obtained PVAc grafted CNT are named F-CNT for short.
After simultaneous irradiation of CNT in monomer solution, the homopolymers were removed by solvent rinsing and F-CNT were obtained (details are presented in ESI†). The high resolution TEM image of F-CNT shows there is an amorphous layer on the surface of the nanotubes, which can be confirmed as the polymer chains of F-CNT (Fig. 1a).21 Fig. 1b shows the FT-IR spectra of CNT and F-CNT. Owing to the defects and residues, there are some intense absorption bands at 2900–3000 cm−1, and <1620 cm−1 in the FT-IR spectrum of CNT.24 After grafting with PVAc chains, several very strong peaks appear at ∼1730 cm−1 (νC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1200 cm−1 (νO–C
O), 1200 cm−1 (νO–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and 1060 cm−1 (νO–C–C), which indicate the existence of PVAc on F-CNT.25 The Raman spectra in Fig. 1c illustrates that grafting of PVAc chains induces much more disorder and defects onto F-CNT, seen from the higher intensity of the D-band in the spectrum of F-CNT compared with that of CNT. The wide XPS spectra of CNT and F-CNT in Fig. 1d show that an intense signal from oxygen appears in the spectrum of F-CNT, but is absent in that of CNT. All these data could confirm the success of PVAc grafting on CNT. The degree of grafting (DG) of F-CNT is determined by thermogravimetric analysis (TGA)23 as presented in Fig. S3.† Here the F-CNT samples labeled as F-CNT-1 correspond to the DG of 2.3%, F-CNT-2 to 7.2% and F-CNT-3 to 13.1%.
O) and 1060 cm−1 (νO–C–C), which indicate the existence of PVAc on F-CNT.25 The Raman spectra in Fig. 1c illustrates that grafting of PVAc chains induces much more disorder and defects onto F-CNT, seen from the higher intensity of the D-band in the spectrum of F-CNT compared with that of CNT. The wide XPS spectra of CNT and F-CNT in Fig. 1d show that an intense signal from oxygen appears in the spectrum of F-CNT, but is absent in that of CNT. All these data could confirm the success of PVAc grafting on CNT. The degree of grafting (DG) of F-CNT is determined by thermogravimetric analysis (TGA)23 as presented in Fig. S3.† Here the F-CNT samples labeled as F-CNT-1 correspond to the DG of 2.3%, F-CNT-2 to 7.2% and F-CNT-3 to 13.1%.
Due to the strong intrinsic van der Waals forces,26 CNT are prone to aggregation and have great trouble dispersing in solvents. PVAc, meanwhile, is a widely-used synthetic polymer with good solubility in organic solvents.27 Therefore, incorporation of PVAc onto CNT is very helpful to enhance their dispersibility in organic solvents, such as acetone, ethyl acetate, tetrahydrofuran, methanol, N-methylpyrrolidone and dimethyl formamide. Here, the F-CNT grafted with PVAc is well dispersed in common organic solvents regardless of how high the DG value is, as shown in Fig. S4.† We dispersed F-CNT in acetone with the assistance of ultrasonication and coated it on a porous PVDF membrane by vacuum-filtration, and obtained an F-CNT/PVDF composite membrane. For contrast, we also prepared a CNT/PVDF composite membrane via the route above.
In Fig. S5,† it can be seen that the former composite membrane is very flat and monolithic, while the CNT/PVDF membrane is lumpy and chapped. That is because the grafting of the PVAc chains has enhanced the dispersibility of the F-CNT in the solvent, reduced its aggregation and made the nanotubes interlace each other in the whole CNT layer of membrane. On the other hand, PVAc also acts as an adhesive to increase the interaction of CNT with each other and with the matrix membrane (i.e. PVDF). Here, we prepare three composite membranes using F-CNTs with different DGs, and named them according to the labeling of F-CNT. That is, F-CNT-1M corresponds to the F-CNT-1 with a DG of 2.3%, F-CNT-2M to the F-CNT-2 with a DG of 7.2% and F-CNT-3M to the F-CNT-3 with a DG of 13.1%.
Interestingly, the CAs of the obtained composite membranes are very high (>150°), meanwhile, water roll-off angles are very low (<5°). As presented in Fig. 2, when a water droplet was dropped on the surface of F-CNT-1M, the CA was measured to be 163.5°. When the membrane is inclined with a slope of 2°, the water droplet starts to roll off (Video S1 and 2 in ESI†). Furthermore, a water droplet sitting on the surface of F-CNT-1M can be completely withdrawn by a syringe needle or absorbed with a tissue paper (Video S3 and 4†), the water drop on syringe needle is very hard to be delivered to the surface of membrane via program controlled stroking (Video S5†). These phenomena indicate the F-CNT/PVDF composite membranes prepared by our route possess good water repellence and superhydrophobicity, which would be very helpful for water desalination by MD.15,28
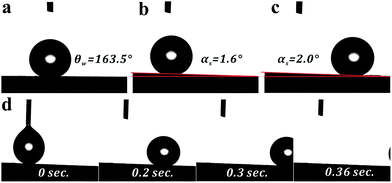 | ||
| Fig. 2 (a) The CA of the F-CNT-1M; (b) no rolling and (c) rolling-off angle of the F-CNT-1M; (d) the fast capture photos of the water drop rolling off of the F-CNT-1M with a slope of 2.0°. | ||
These composite membranes have been equipped for DCMD, and assessed by evaluating the permeate flux through the membrane, the salt rejection capability and the performance stability. The details of operation and calculation are presented in the experimental part of the ESI.† Fig. 3a shows the flux values of composite membranes tested by pure water feeding are decreased in comparison to those of the bare PVDF membrane. When feeding 3.5 wt% NaCl solutions, the flux values of F-CNT-1M and F-CNT-2M are very close to that of the bare PVDF membrane, which is up to 20 kg m−2 h−1 at 70 °C,29 but the values for F-CNT-3M are still much lower than those of other membranes (Fig. 3b). Fig. 3c shows that the salt rejection of the bare PVDF membrane is not sufficient. When the inlet temperature is 70 °C, the salt rejection reaches only ∼98% for the bare PVDF membrane, while the salt rejection values of those composite membranes are all greater than 99.5%. These results indicate that the excellent superhydrophobicity of the F-CNT membranes does provide an improvement in the salt rejection.
The stability in the DCMD performance of the different composite membranes is investigated and compared with that of the bare PVDF membrane. The results show that the stability of the DCMD performance is strongly dependent on the DG of the F-CNT, in other words, on the amount of PVAc graft chains attached on the CNT. For example, the salt rejection of F-CNT-1M decreased suddenly after running for 4 hours (Fig. S6a†) and some black solid was observed in the circulating feed solution during DCMD testing. These phenomena didn't happen in the testing of the F-CNT-2M and F-CNT-3M. It indicates that F-CNT with a low DG is not stable and is prone to delamination during MD operation. This could be attributed to the poor interaction of the F-CNT with each other due to fewer inter-chain interactions between the PVAc graft chains. Fig. 3d shows that the flux and salt rejection of F-CNT-2M do not experience any obvious decay even after 10 hours of testing. Although the performances of F-CNT-3M (Fig. S6b†) are also stable during the whole testing, its flux is just half that of F-CNT-2M. Therefore, F-CNT-2M is the optimal candidate for water desalination under DCMD mode. We have measured the flux and salt rejection of F-CNT-2M by feeding 3.5 wt% and 10 wt% NaCl solution. Fig. 3e reveals that F-CNT-2M has good permeate flux values: up to 25 and 20 kg m−2 h−1 for 3.5 wt% and 10 wt% NaCl solutions at 80 °C, respectively. Also, the excellent salt rejection, greater than 99.5%, for F-CNT-2M at different salt concentrations and temperatures can be found in Fig. 3f. By comparing the water distillation performance of F-CNT-2M with other reported membranes in Table S1† (ref. 17, 30 and 31) and some commercial flat sheet membranes summarized by M. Khayet et al.,31 we can make a conclusion that F-CNT-2M is suitable for the DCMD process, because of its high permeate flux and salt rejection rate.
Fig. 4 shows the cross-section features of F-CNT-1M, F-CNT-2M and F-CNT-3M characterized by FE-SEM. It can be seen from the image of F-CNT-1M that the F-CNT layer is thick but very fluffy (Fig. 4a and b), while for F-CNT-3M, it is thinner and more compact (Fig. 4e and f). These could be attributed to the different amounts of PVAc grafted on F-CNT. The adhesive force of F-CNT with low DG is weaker, within itself and with the PVDF surface, as compared to that with high DG. Therefore, it appears loose and easily washed off from the PVDF membrane by a hot feed solution, which leads to a decrease in the performance stability. On the other hand, at high DG, the adhesive force is greater. F-CNT could be self-assembled closely and tightly cemented on the PVDF membrane surface. As a result, the porosity of F-CNT-3M was decreased and the flux subsequently decreased as shown in Fig. 3a and b. The surface microtopography of the membranes also illustrates this conclusion, that the degree of compactness of the F-CNT coating on the PVDF membrane is proportional to the DG (Fig. S7†). In other words, a high DG improves the structural stability of the composite membrane.
 | ||
| Fig. 4 The cross-section SEM images of F-CNT-1M (a) and (b); F-CNT-2M (c) and (d) and F-CNT-3M (e) and (f). | ||
However, a high DG is not conducive to obtain and maintain the superhydrophobicity of F-CNT/PVDF composite membranes. We measured the water contact angle of F-CNT-1M, F-CNT-2M and F-CNT-3M before and after DCMD testing. We found that the water contact angles of F-CNT-1M and F-CNT-2M are >150° before and after 2 hours of DCMD testing, while F-CNT-3M is not superhydrophobic, not only before but also after DCMD testing, and its water contact angle shows a very obvious decrease (Fig. S8†). This phenomenon may be due to the fact that a large amount of PVAc alters the surface chemical energy of the MWNT to be similar to that of a pure PVAc surface. In order to clarify this, we have measured the water CA of a pure PVAc film prepared by casting PVAc solution on a glass slide surface, obtained via radiation induced polymerization of vinyl acetate under the same conditions as the grafting process. We find the water CA of the pure PVAc film is only about 92.3° (Fig. S9†), which is very close to that of F-CNT-3M. According to these results, we can conclude that the DG of PVAc on F-CNT is critical to the performance of composite membranes.
In summary, modifying a commercial PVDF membrane with PVAc functionalized CNT is a facile approach to obtain superhydrophobicity and outstanding MD performance. These modified PVDF membranes have been tested on a DCMD setup, and have shown high salt rejection (>99.5%) and considerable flux (20 kg m−2 h−1). After MD testing, the water contact angle of the composite membranes remained greater than 150°. Additionally, the microstructure and porosity of the F-CNT coating on the PVDF membrane can be adjusted by altering the DG of F-CNT, which determines the MD performance stability of composite membranes.
Acknowledgements
This work was supported by the National High-Tech R&D Program of China (2012AA021202), the National Natural Science Foundation of China (11175234, 11105210, 11305241 and 11305248), the “Strategic Priority Research Program” of the Chinese Academy of Sciences (XDA02040300), the Solar Energy Initiative of the Chinese Academy of Sciences (KGCX2-YW-380), the “Knowledge Innovation Program” of the Chinese Academy of Sciences (KJCX2-YW-N49), and the Shanghai Municipal Commission for Science and Technology (12ZR1453300).Notes and references
- U./WHO, Progress on Drinking Water and Sanitation, 2012, pp. 1–58 CrossRef CAS PubMed; P. Aldhous, Nature, 2003, 422, 251 CrossRef CAS PubMed; N. Gilbert, How to avert a global water crisis, http://www.nature.com/news/2010/<?ccdc_no 101004?>101004<?ccdc END?>/full/news.2010.490.html.
- K. Than, Could Seawater Solve the Freshwater Crisis?, http://news.nationalgeographic.com/news/2011/08/110804-fresh-water-crisis-desalination-environment-science/; D. El-Akkad, Cheap desalination membranes may help Egypt overcome water shortage, http://www.nature.com/nmiddleeast/2013/<?ccdc_no 130603?>130603<?ccdc END?>/full/nmiddleeast.2013.83.html.
- M. Elimelech and W. A. Phillip, Science, 2011, 333, 712–717 CrossRef CAS PubMed; Y. T. Chua, C. X. C. Lin, F. Kleitz, X. S. Zhao and S. Smart, Chem. Commun., 2013, 49, 4534–4536 RSC; C. Forrestal, P. Xu and Z. Ren, Energy Environ. Sci., 2012, 5, 7161–7167 Search PubMed; S.-I. Jeon, H.-R. Park, J.-G. Yeo, S. Yang, C. H. Cho, M. H. Han and D. K. Kim, Energy Environ. Sci., 2013, 6, 1471–1475 Search PubMed; M. M. Pendergast and E. M. V. Hoek, Energy Environ. Sci., 2011, 4, 1946–1971 Search PubMed; S. Porada, B. B. Sales, H. V. M. Hamelers and P. M. Biesheuvel, J. Phys. Chem. Lett., 2012, 3, 1613–1618 CrossRef; D. Cohen-Tanugi and J. C. Grossman, Nano Lett., 2012, 12, 3602–3608 CrossRef PubMed.
- M. A. Shannon, P. W. Bohn, M. Elimelech, J. G. Georgiadis, B. J. Marinas and A. M. Mayes, Nature, 2008, 452, 301–310 CrossRef CAS PubMed.
- D. Li and H. T. Wang, J. Mater. Chem., 2010, 20, 4551–4566 RSC; A. M. Alklaibi and N. Lior, Desalination, 2005, 171, 111–131 CrossRef CAS PubMed.
- J.-P. Mericq, S. Laborie and C. Cabassud, Water Res., 2010, 44, 5260–5273 CrossRef CAS PubMed.
- K. W. Lawson and D. R. Lloyd, J. Membr. Sci., 1997, 124, 1–25 CrossRef CAS.
- A. Alkhudhiri, N. Darwish and N. Hilal, Desalination, 2012, 287, 2–18 CrossRef CAS PubMed.
- M. Gryta, J. Grzechulska-Damszel, A. Markowska and K. Karakulski, J. Membr. Sci., 2009, 326, 493–502 CrossRef CAS PubMed; M. Gryta, Chem. Pap., 2012, 66, 92–98 CrossRef PubMed.
- G. A. Mannella, V. La Carrubba and V. Brucato, J. Appl. Polym. Sci., 2011, 122, 3557–3563 CrossRef CAS PubMed.
- W. L. Min, B. Jiang and P. Jiang, Adv. Mater., 2008, 20, 3914–3918 CrossRef CAS PubMed; N. J. Shirtcliffe, G. McHale, S. Atherton and M. I. Newton, Adv. Colloid Interface Sci., 2010, 161, 124–138 CrossRef PubMed; B. Deng, R. Cai, Y. Yu, H. Jiang, C. Wang, J. Li, L. Li, M. Yu, J. Li, L. Xie, Q. Huang and C. Fan, Adv. Mater., 2010, 22, 5473–5477 CrossRef PubMed; A. Marmur, Soft Matter, 2013, 9, 7900–7904 RSC.
- Z. Ma, Y. Hong, L. Ma and M. Su, Langmuir, 2009, 25, 5446–5450 CrossRef CAS PubMed; Y. Liao, R. Wang and A. G. Fane, J. Membr. Sci., 2013, 440, 77–87 CrossRef PubMed.
- J. Zhang, Z. Song, B. Li, Q. Wang and S. Wang, Desalination, 2013, 324, 1–9 CrossRef CAS PubMed.
- J. Cho, H. Nam, H. Ryu and G. Lim, Adv. Funct. Mater., 2013, 23, 5577–5584 CrossRef PubMed.
- J. H. Zhang, N. Dow, M. Duke, E. Ostarcevic, J. D. Li and S. Gray, J. Membr. Sci., 2010, 349, 295–303 CrossRef CAS PubMed.
- C. H. Lee, N. Johnson, J. Drelich and Y. K. Yap, Carbon, 2011, 49, 669–676 CrossRef CAS PubMed; C. Lee and S. Baik, Carbon, 2010, 48, 2192–2197 CrossRef PubMed.
- L. F. Dumee, K. Sears, J. Schutz, N. Finn, C. Huynh, S. Hawkins, M. Duke and S. Gray, J. Membr. Sci., 2010, 351, 36–43 CrossRef CAS PubMed.
- Y. Liu, J. Tang, R. Wang, H. Lu, L. Li, Y. Kong, K. Qi and J. H. Xin, J. Mater. Chem., 2007, 17, 1071–1078 RSC.
- G. Li, H. Wang, H. Zheng and R. Bai, Langmuir, 2010, 26, 7529–7534 CrossRef CAS PubMed.
- M. Yu, Z. Q. Wang, H. Z. Liu, S. Y. Xie, J. X. Wu, H. Q. Jiang, J. Y. Zhang, L. F. Li and J. Y. Li, ACS Appl. Mater. Interfaces, 2013, 5, 3697–3703 Search PubMed; Y. Yu, B. W. Zhang, M. Yu, B. Deng, L. F. Li, C. H. Fan and J. Y. Li, Sci. China: Chem., 2012, 55, 2202–2208 CrossRef CAS PubMed; H. J. Ma, H. Y. Chi, J. X. Wu, M. Wang, J. Y. Li, H. Hoshina, S. Saiki and N. Seko, ACS Appl. Mater. Interfaces, 2013, 5, 8761–8765 Search PubMed.
- H. X. Xu, X. B. Wang, Y. F. Zhang and S. Y. Liu, Chem. Mater., 2006, 18, 2929–2934 CrossRef CAS; S. M. Chen, G. Z. Wu, Y. D. Liu and D. W. Long, Macromolecules, 2006, 39, 330–334 CrossRef.
- R. Cai, B. Deng, H. Q. Jiang, Y. Yu, M. Yu, L. F. Li and J. Y. Li, Radiat. Phys. Chem., 2012, 81, 1354–1356 CrossRef CAS PubMed; J. X. Wu, J. Y. Li, B. Deng, H. Q. Jiang, Z. Q. Wang, M. Yu, L. F. Li, C. Y. Xing and Y. J. Li, Sci. Rep., 2013, 3, 2951 Search PubMed.
- B. W. Zhang, Y. J. Zhang, C. Peng, M. Yu, L. F. Li, B. Deng, P. Hu, C. H. Fan, J. Y. Li and Q. Huang, Nanoscale, 2012, 4, 1742–1748 RSC.
- L. Stobinski, B. Lesiak, L. Kövér, J. Tóth, S. Biniak, G. Trykowski and J. Judek, J. Alloys Compd., 2010, 501, 77–84 CrossRef CAS PubMed.
- H. Jeon, J. Youk and W.-R. Yu, Macromol. Res., 2010, 18, 458–462 CrossRef CAS PubMed.
- L. A. Girifalco, M. Hodak and R. S. Lee, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 62, 13104 CrossRef CAS.
- H. Y. Erbil, in Vinyl Acetate Emulsion Polymerization and Copolymerization with Acrylic Monomers, CRC Press, 2000 Search PubMed.
- M. Gryta, Membranes, 2012, 2, 415–429 CrossRef CAS.
- L. Camacho, L. Dumée, J. Zhang, J.-D. Li, M. Duke, J. Gomez and S. Gray, Water, 2013, 5, 94–196 CrossRef.
- B. S. Lalia, E. Guillen-Burrieza, H. A. Arafat and R. Hashaikeh, J. Membr. Sci., 2013, 428, 104–115 CrossRef CAS PubMed; J. A. Prince, G. Singh, D. Rana, T. Matsuura, V. Anbharasi and T. S. Shanmugasundaram, J. Membr. Sci., 2012, 397–398, 80–86 CrossRef PubMed; C. Yang, X.-M. Li, J. Gilron, D.-F. Kong, Y. Yin, Y. Oren, C. Linder and T. He, J. Membr. Sci., 2014, 456, 155–161 CrossRef PubMed; Y. Liao, R. Wang, M. Tian, C. Qiu and A. G. Fane, J. Membr. Sci., 2013, 425–426, 30–39 CrossRef PubMed; L. F. Dumée, K. Sears, J. Schütz, N. Finn, C. Huynh, S. Hawkins, M. Duke, S. Gray, L. Dumée, V. Germain, K. Sears, J. Schütz, N. Finn, M. Duke, S. Cerneaux, D. Cornu and S. Gray, J. Membr. Sci., 2011, 376, 241–246 CrossRef PubMed; L. Dumée, J. L. Campbell, K. Sears, J. Schütz, N. Finn, M. Duke and S. Gray, Desalination, 2011, 283, 64–67 CrossRef PubMed; L. Dumée, K. Sears, J. R. Schütz, N. Finn, M. Duke and S. Gray, Desalin. Water Treat., 2010, 17, 72–79 Search PubMed.
- M. Essalhi and M. Khayet, J. Membr. Sci., 2013, 433, 167–179 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3ra47436d |
| ‡ Dr B. W. Zhang and Miss L. X. Liu contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2014 |


